the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
A dynamical process-based model for quantifying global agricultural ammonia emissions – AMmonia–CLIMate v1.0 (AMCLIM v1.0) – Part 2: Livestock farming
David S. Stevenson
Aimable Uwizeye
Giuseppe Tempio
Alessandra Falcucci
Flavia Casu
Mark A. Sutton
Agricultural ammonia (NH3) emissions are a major pathway of nitrogen loss, which can have significant environmental consequences, such as air and water pollution, ecosystem damage, and biodiversity loss. Ammonia emissions related to livestock farming are major sources in the agricultural sector, resulting from animal housing, manure management and land application. This paper is the second part of the description of the AMmonia–CLIMate (AMCLIM) model, presenting the development and application of all three main modules to estimate NH3 emissions from livestock, including pigs, poultry (chickens), cattle, sheep and goats. The AMCLIM model simulates the flows of N species at different stages of livestock agriculture. It incorporates the effects of environmental factors and also provides an adequate level of detail for the representation of human management practices. According to simulations by AMCLIM, it is estimated that NH3 emissions from global livestock farming are about 29.9 Tg N yr−1, accounting for around 30 % of total excreted nitrogen. Cattle and buffalo systems are estimated to be the largest sources of NH3 emissions, contributing over 60 % of total livestock emissions. Both pig and poultry systems result in more than 15 % of estimated total emissions, while sheep and goats are responsible for the remaining 7 %. High volatilization rates frequently occur in hot regions, indicating the climate-dependence of NH3 volatilization. It is also shown how AMCLIM can simulate the influence of management practices on NH3 volatilization, e.g. illustrating how fully enclosed animal houses with heating and forced ventilation can result in higher emissions than naturally ventilated barns, while poorly managed manure leads to substantially increased NH3 emissions.
- Article
(38601 KB) - Full-text XML
- Companion paper
-
Supplement
(1044 KB) - BibTeX
- EndNote
Ammonia (NH3) is the primary form of reduced reactive nitrogen (Nr) and mainly originates from agricultural activities. Excessive NH3 emissions affect air, soil and water quality; impact local ecosystems and biodiversity; and can pose serious threats to human society. At the same time, NH3 volatilization is one of the key pathways of N leak from agricultural systems to the environment, representing critical nutrient loss and causing an unnecessary economic cost.
Livestock farming is an important component of agricultural systems. As the global population grows, livestock numbers have increased dramatically to fulfil the rising demand for animal products such as milk, meat and eggs. Specifically, pigs and poultry are the sectors that have recorded the largest increase in livestock population numbers, with pigs having increased by about 140 % and poultry having increased by nearly 5-fold over the past 50 years (FAO, 2022). This surge in the livestock population has also resulted in a substantial increase in nutrient requirements, particularly in N inputs in animal feed. However, N recycling within livestock farming systems is often poor, resulting in a significant amount of N loss (rather than N being used by the animals). In particular, NH3 emissions are a major pathway of N loss to the environment and can cause serious environmental problems (Sutton et al., 2011). Therefore, accurate estimation of NH3 emissions is crucial for assessing the environmental impact of livestock farming systems and optimizing resource utilization.
Cattle systems contribute the largest amount of NH3 emissions among livestock (Uwizeye et al., 2020). Existing studies have reported that over 50 % to 60 % of animal-related NH3 originates from cattle agriculture (including buffaloes), while sheep and goat farming together resulted in around 10 % of livestock NH3 emissions (Behera et al., 2013; Bouwman et al., 1997; Dentener and Crutzen, 1994). According to FAO statistical data, ruminant populations have increased by more than 60 % over the past 50 years (FAO, 2022). Compared with pigs and poultry, NH3 can also originate from excreted nitrogen during ruminant grazing, which is still poorly quantified globally and needs to be investigated.
The most commonly employed method for estimating NH3 emissions is using emission factors (EFs), combined with statistical activity data for different source sectors. However, as NH3 emissions are highly sensitive to environmental conditions, such as temperature and water availability, EFs usually only consider the climatic effects to a limited extent, so they may not accurately represent NH3 volatilization. To address this deficiency of EFs, process-based models are developed based on the theoretical understanding of relevant processes (Flechard et al., 2013; Móring et al., 2016; Nemitz et al., 2001; Sutton et al., 1995). A challenge for process-based models is the representation of the various management practices existing in livestock agriculture, which can also influence the NH3 volatilization in different ways. Such complications are difficult to parameterize in models while also maintaining the consistency of the model structuring, especially for large-scale simulations. Other barriers for the modelling include the high requirement with respect to input data and the shortage of sufficient-quality observations for validation and evaluation.
A process-based, dynamical emission model, AMmonia–CLIMate (AMCLIM), has been specifically designed that incorporates the effects of both environmental conditions and the management practice to simulate agricultural NH3 emissions. Compared with existing process-based models, AMCLIM is thought to be the first model that simulates NH3 emission from both synthetic fertilizer use and livestock farming using a consistent process-based modelling approach, with high levels of detail with respect to the representation of agricultural practices. Other process-based models exist, such as the “Flow of Agricultural Nitrogen model version 2” (FANv2; Vira et al., 2020), which simulates agricultural NH3 emissions interactively within the Community Earth System Model (CESM) with detailed soil processes for land application of fertilizers and ruminant grazing, or the “Calculation of AMmonia Emissions in ORCHIDEE” (CAMEO) model, which includes several management modules for livestock feed, manure management and agricultural handling practices within a global land surface model (Beaudor et al., 2023). However, while these models still largely rely on EFs for estimating NH3 emissions from livestock sectors, AMCLIM explicitly models the N flows within the systems and includes several major N processes. AMCLIM uses an integrated approach to simulate how various N species are influenced by environmental factors in a sequence of practices in the livestock sector, from livestock housing to manure management and ultimate application of manure to fields, as well as ruminant grazing. By following this sequence in AMCLIM, changes in emissions at an early stage of livestock agriculture influence the simulated N pools and can thereby affect emission at a later stage of these activities.
The structure and simulations of AMCLIM for global synthetic fertilizer use have been presented in a companion paper (Jiang et al., 2024). In the present paper, the development of the modules, evaluation and application of the AMCLIM model for simulating NH3 emissions from livestock farming are described. An earlier version of this conceptual approach has already been reported by Jiang et al. (2021) with a focus on chicken farming. The present paper describes (1) the development of the approach for other livestock and (2) updates in relation to the treatment for poultry. The AMCLIM model has been tested against measurements at a site scale and then applied at the global scale.
2.1 AMCLIM model structure
The design of the AMCLIM model is closely associated with human activities in agriculture systems. The model structure and components are shown in Fig. 1 (same as Fig. 1 in the companion paper, Jiang et al., 2024). There are three modules in AMCLIM: (a) housing, (b) manure management and (c) land. The development and application of the land module (AMCLIM–Land) for simulating synthetic fertilizer use has been described in detail in Jiang et al. (2024). Therefore, the present paper mainly focuses on the livestock sector, including pigs, poultry (chickens) and major ruminants (cattle, sheep and goats).
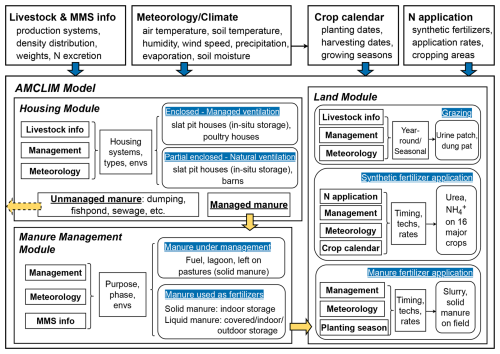
Figure 1Components and structure of the AMCLIM model and inputs (blue arrows) used for simulations. The dashed yellow arrows represent a fraction of unmanaged N from housing that is not simulated in the manure management module (AMCLIM–MMS). Solid yellow arrows represent the N flows between modules (MMS: manure management system; Envs: environments; Techs: techniques).
Livestock consume N from feed crops, agrifood industry by-products and concentrated feed for gaining weight and producing meat, milk and eggs. Most N ingested through feeding is excreted through urine and dung, and this excreted N can be a valuable source of organic fertilizer for grassland and cropland application. The animal excreta collected from animal houses is stored as slurry or solid manure and then applied to arable land during growing seasons. However, the management of livestock manure can vary greatly across regions. For example, some farmers spread manure daily or simply leave it in the yard or holding area without much management or storage. Each of these management practices can result in NH3 emissions.
All three modules in AMCLIM are operated for capturing the activities and practices of livestock farming. The connections between modules reflect typical N flows in the livestock production systems, from animal housing to manure storage/management and then to the ultimate land application, as shown in Fig. 1. Because NH3 emissions can be released at all stages, all three modules need to provide robust estimates, as previous components can have substantial influences on the following ones; i.e. less emission from housing leaves a higher N content in the animal excreta, which can cause larger emissions in the succeeding practices. The following sections describe the different modules that are used to address these components as well as how the modules are linked. The focus here is on describing the housing (AMCLIM–Housing) and manure management (AMCLIM–MMS) modules of AMCLIM. This paper highlights the different processes of manure application compared to synthetic fertilizer application and differentiates the processes specific to grazing livestock.
2.2 Housing module of AMCLIM: AMCLIM–Housing
2.2.1 Housing systems and house types
The housing module in AMCLIM (AMCLIM–Housing) was designed to estimate the NH3 emissions from livestock housing using principles relevant for different livestock types. Pigs and poultry are mostly kept in buildings, while ruminants like cattle and sheep may also spend a considerable amount of time in barns or stalls depending on the weather and local management. In each case, NH3 emissions are the result of the decomposition of excreted N, with negligible amounts (by comparison) assumed to be emitted through animal breath and sweat.
AMCLIM–Housing includes two housing systems and three house types, depending on the livestock production system and management. Two housing systems are distinguished: enclosed housing and partially enclosed housing, which are reflected by different indoor environmental conditions. Enclosed housing is assumed to have forced heating and managed ventilation, which is commonly used for commercial pigs and poultry in order to improve livestock performance (Gyldenkærne, 2005; FAO, 2018). Partially enclosed housing refers to barns or houses that are naturally ventilated, the indoor environment of which is assumed to be close to the natural environment. These two systems are employed to differing degrees by different livestock sectors and production systems. For example, cattle have a higher tolerance to cold weather than pigs and poultry, so they are typically kept in naturally ventilated barns (Seedorf et al., 1998).
The three house types in AMCLIM–Housing include the following: (1) houses with slatted floors and storage pits, (2) normal barns (without slatted floors and pits), and (3) deep-litter poultry houses. The first house type with slatted floors allows animal excreta to be removed quickly and effectively, so the house can be easily cleaned. The slatted floor is usually concrete or iron, and there are partially slatted compartments. The gap area of the slatted floor usually accounts for approximately 20 % and no more than 50 % of the total floor area (Aarnink et al., 1997). The excreta falls to the pit underneath through the gaps and is stored in situ for a period. Emission of NH3 can be from both the slatted floor area and the storage pit. Such slatted pit houses are prevalent in pig farming, especially for industrial production systems. A two-reservoir emission scheme is used for this type of housing in AMCLIM–Housing, with the pit storage simulated by a two-film model (Liss, 1973; Liss and Slater, 1974). The two-reservoir emission scheme details are given in Sect. 2.2.3, and the two-film model is described in Sect. S3.1.
The second house type is barns. Barns are commonly used facilities in livestock housing because they can be easily set up and require less capital input compared to animal houses with slatted floors and pit storage. Barns are normally naturally ventilated and are not fully enclosed. On cold days, mechanical blocking may be applied to open barns to reduce ventilation (Gyldenkærne, 2005). Excreta and bedding are frequently removed to a separate storage unit to keep the barn clean. In most cases, daily cleaning of barns is necessary.
The third house type is deep-litter poultry houses. Except for some regions, poultry houses for broiler and layer production systems are mainly enclosed with forced heating and ventilation. Commonly, poultry excreta accumulates and remains in the houses for a long time, e.g. months to years, until it is removed. Bedding materials, such as straw, are added to absorb moisture and to reduce emissions, which is a typical management practice for breeder and broiler systems (FAO, 2018).
Across the world, there are many other variants of animal housing systems. However, the major systems that are listed can be considered sufficient for the focus of exploring the sensitivity of climate to NH3 emissions, while providing a modelling approach that addresses the major management opportunities to reduce emissions.
2.2.2 Simulated processes in animal houses
Animal housing is one of the primary sources and often the very first origin of NH3 emissions in livestock farming systems. Figure 2 depicts the processes through which NH3 emissions originate from excreta in animal houses, ultimately released into the outdoor atmosphere. In general, there are six processes that can be summarized:
-
Process 1: excretion. Livestock excreta contains N in the form of urea in pig and ruminant urine (and uric acid in poultry excretion) as well as other organic forms of N in pig and ruminant dung and poultry faeces.
-
Process 2: conversion of excreted N to ammoniacal N. Excreted N on the floor surface of the animal house is converted to total ammoniacal nitrogen (TAN) through the hydrolysis of urea or uric acid and the decomposition of organic N (details are given in Sect. S2).
-
Process 3: equilibration of TAN. The TAN pool partitions into multiple phases; gaseous NH3 is in equilibrium with aqueous TAN.
-
Process 4: emission from surfaces. Ammonia volatilizes to the house atmosphere from manure and other surfaces in the building.
-
Process 5: accumulation of gaseous ammonia. The indoor NH3 level builds up due to NH3 volatilization according to the limited extent of ventilation.
-
Process 6: emission to outside the building. Indoor NH3 is removed from the house to the outside atmosphere through ventilation.
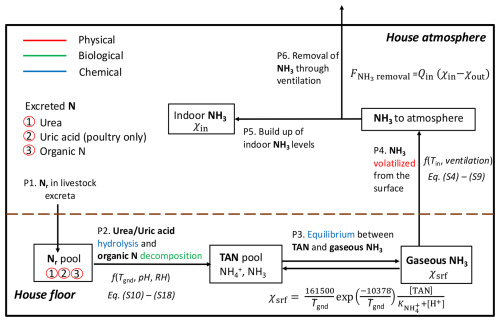
Figure 2Schematic of NH3 volatilization in animal houses (adapted from Elliott and Collins, 1982; Jiang et al., 2021). Physical, biological and chemical processes are highlighted in red, green and blue, respectively.
The concentration of NH3 inside the animal house (χin, g m−3) is regulated by the balance between NH3 volatilization from the floor surface () and removal of NH3 to the outside atmosphere (), which can be expressed by the following equation:
where the fluxes are expressed as the sum of the flux for the whole animal house (g N s−1). The time-dependent concentration of indoor NH3 of the animal house can be represented by the following equation:
where χin (g m−3) represents the indoor NH3 concentration assuming a well-mixed state of air inside the animal house. χsrf (g m−3) is the gaseous NH3 concentration at the emitting surface, and χout (g m−3) is the free-atmosphere NH3 concentration. Shouse (m2) and Vhouse (m3) represent the surface area and the volume of the house, respectively. Qin (m3 s−1) is the airflow rate of the house. The resistance for NH3 volatilization in the animal house (RG,house, s m−1) is determined by the inverse of an empirically derived gaseous transfer coefficient for NH3(kG,housing, m s−1), which depends on housing conditions such as temperature and ventilation, as expressed by the following equation:
Animal houses are cleaned after a certain amount of time. The frequency of cleaning varies depending on the housing management. The TAN pool (MTAN, given per unit area; all masses have units of g m−2 if not specifically explained) in the animal house can be determined by the following equation:
where FTAN is the TAN production, i.e. through urea or uric acid hydrolysis and the decomposition of organic N for livestock excreta (together with other processes, as presented in Sect. S2). is the flux of NH3 volatilization (all following N fluxes/flows have units of g N m−2 s−1 if not specifically explained). ψcleaning(t) represents the cleaning event of the house and is expressed as follows:
The cleaning event refers to the removal of livestock excreta (Mexcreta), all N species (MN) and water () from the animal house within an assumed timescale of 24 h (tcleaning).
The removed excreta can either be stored or applied to land as fertilizer, which will be described in the following sections. The pools for other N species, e.g. urea, in the animal houses can be expressed as follows:
where FexcretN is the total N excretion rate from the livestock and fN is the fraction of an N form in the excretion. KN is the conversion rate (s−1) at which an N species (MN) decomposes. For pigs and ruminants, nitrogen is excreted in AMCLIM as urinary N and faecal N, with urinary N being in the form of urea and organic forms (Jørgensen et al., 2013; Vu et al., 2009a, b). For poultry, AMCLIM assumes that 60 % of the excreted N is in the form of uric acid, whereas the remaining 40 % is in organic forms (Nahm, 2003). The excretion pool is determined using the following equation:
where Fexcreta is the excretion rate from the livestock, which is derived from the N excretion rates based on the N content in the excreta. The pH of the livestock excretion is used for determining the decomposition rates of N species and chemical equilibria in housing simulations. As discussed in a companion paper (Jiang et al., 2024), substrate pH is a critical factor that impacts NH3 emission. The dynamic equilibrium between gaseous NH3 and aqueous ammonium is dependent on pH. On the other hand, pH affects the rates of uric acid hydrolysis and nitrification, which together control the TAN pool. In AMCLIM, the pH of livestock excretion is used for determining the decomposition rates of N species and chemical equilibria in housing simulations.
It is worth noting that there are different characteristics for the simulations of animal housing between the studied livestock sectors, which are presented in Sect. S3.
2.2.3 Two-reservoir emission scheme for simulating houses with slats and pits
Houses with slatted floor and pit storage allow animal excreta to be stored in situ, keeping the floor area clean. For this housing type, a two-source emission scheme is used to model NH3 emissions, as there are two emitting surfaces: the slats and the pit. The two NH3 emission elements are treated as additive, i.e. the total housing emission is the sum of the emissions from the two housing compartments. The pools of N species and other simulated variables are divided into two separate reservoirs to represent the processes on the slats and in the pit. Livestock excreta is split proportionally between the two reservoirs depending on the gap space of the slats. For example, if the gap space is 20 % and the slat space is 80 %, 20 % of initial pig excreta will fall into the pit, whereas the remaining 80 % will stay on the slats. Given the fact that excreta left on the slats will eventually fall to the pit (i.e. through cleaning) but excreta in the pit cannot go back to the slatted floor above, a unidirectional transfer is applied daily in AMCLIM–Housing. It is assumed that all pools from the slat reservoir go into pit reservoir by the end of each day, and the slat reservoir is subsequently reset to zero. Excreta that goes into the pit is stored for longer, e.g. weeks to months.
The process of NH3 volatilization differs between the two reservoirs because of the different amount of water held in each. For the slats, excreta is typically a thin, wet layer, so the surface concentration can be expressed by the concentration of the entire layer. The gaseous NH3 concentration at the surface is directly derived from the aqueous TAN concentration of this layer. In contrast, the pit reservoir holds more water (and faeces) because urine in the excreta accumulates in the pit. There is an additional aqueous transfer process of TAN from the bulk water to the air–water interface. As described in Sect. S1, AMCLIM–Housing incorporates a two-film model that describes the gas exchange across the air–liquid interface (Liss, 1973; Liss and Slater, 1974).
2.3 Manure management module of AMCLIM: AMCLIM–MMS
2.3.1 Manure management systems
Properly dealing with animal excreta is crucial, as poorly managed animal excreta can cause large, unintentional N losses due to NH3 emission. Under adequate management, livestock excreta is a valuable N source as a fertilizer. Manure is a mixture of animal excreta (including urine and faeces), bedding, feed, drinking water and water used for cleaning from the housing. Collected manure is usually stored for a period before it is applied to fields at an appropriate time, and manure can also be used as fuel.
The manure management module (AMCLIM–MMS) was developed to simulate the NH3 emission from the stage after manure is removed from the housing systems and before it is spread on land. The Global Livestock Environmental Assessment Model (GLEAM, https://www.fao.org/gleam/en/, last access: 3 August 2023) considers over 20 manure management systems (MMSs) (Uwizeye et al., 2020), with manure in either a liquid or solid phase depending on the water content. The main divisions identified for AMCLIM and regrouped from the MMSs defined in GLEAM are based on the following similarities existing in the general practices:
- a.
Indoor storage. Manure is stored and managed in stables/barns/enclosed or partially enclosed facilities.
- b.
Outdoor storage. Manure is stored in open environments, i.e. an earthen basin or pond.
- c.
Covered storage. Manure is stored in tanks or containers with a cover/crust on top.
- d.
Left on land. Manure is left on pastures soon after it is removed from housing or there is daily spreading of collected manure on fields.
The above divisions of MMSs were implemented in AMCLIM–MMS for simulating NH3 emissions, and each division may include one or two phases. It is worth emphasizing that the types of manure storage included in the model are a simplification. The current level of complexity is justified as adequate for large-scale/global modelling, as it is unrealistic to simulate every specific practice in manure management given the computational costs and the additional uncertainty incurred from more assumptions on data and processes. AMCLIM represents divisions A, B and C of manure storage in different manners. Subsequent land spreading of manure N from these three divisions is simulated by the AMCLIM land module. By comparison, manure N from division D which has already been spread or left on land is not subsequently passed to the land module of AMCLIM, and the NH3 emission is counted as manure management emission. As described in Table A1, manure can be used as fuel (burned) or converted to fuel (digester), which may cause significant NH3 emissions, but this is not included in the AMCLIM model due to the uncertainty (limited studies) and the fact that it is outside the scope of this study. Meanwhile, these types of management are only a small fraction across the globe. The amount of manure N used as fuel is not simulated further. In addition, there is unmanaged manure N from housing; although this is not an MMS according to the definitions used in GLEAM, it is still critical, as this fraction reflects a direct N loss from the agricultural system to the environment. According to FAO (2018), unmanaged N is quite common in a few regions and nations for some particular livestock production systems. These systems include “discharge”, “dumping”, “fishpond” and “public sewage”, which have adverse impacts on local aquatic systems and ecosystems. Manure illegally discharged to waterbodies is expected to contribute much lower NH3 emissions (because of dilution), but it has other environmental implications (e.g. eutrophication). Manure N not managed at this stage is reported, but it is not simulated further in AMCLIM. This is treated as a loss or an untraceable term in AMCLIM–MMS.
2.3.2 Simulated processes in manure management systems
The AMCLIM model simulates manure management for livestock as a subsequent stage after housing, except in situ storage of livestock excreta in pits or litter management for poultry, which are counted as part of the housing emissions. In AMCLIM–MMS, there are two types of manure under management: slurry and solid manure, corresponding to liquid manure and manure with a mixture of solid and liquid phases, respectively.
Liquid manure or slurry can have a dry matter (DM) content that ranges from 2 % to 20 %, depending on the amount of water added to the manure. As such, slurry refers to manure with a relatively low DM content, which consists mainly of urine, faeces and added water (Sommer et al., 2006; Vira et al., 2020). In this study, “liquid manure” used in the manure management section is the same as “slurry” in the land application section. Figure 1 illustrates the three types of storage for liquid manure: indoor, outdoor and covered storage.
The indoor storage of liquid manure and pit storage in animal houses are similar, as both reservoirs have a high water content (although, as mentioned, it should be noted that NH3 emissions from pits in animal houses are included in the housing emissions). The volatilization of NH3 from indoor storage of liquid manure is calculated using the same method as for pit emissions (two-film model; see Sect. S1). The TAN pool of the storage unit is determined from the TAN pool from housing, conversion from other N species, loss through NH3 volatilization and removal when manure is used for land application, which can be expressed by the following equation:
where ψhousing(t) is the function that represents the housing excreta that is transferred to the storage unit. The relationship between ψhousing(t) and the cleaning function ψcleaning(t) can be expressed as follows:
where fstore-housing is the ratio of storage area to housing area. If the area for manure storage is smaller than the housing area, the pools of manure storage (per unit area) will be larger than housing, as manure concentrates in smaller areas (note that concentrations remain unchanged). The function ψto land(t) represents stored manure used for land application within 24 h (tto land) and is expressed as follows:
Similarly, the other N pools during storage can be expressed as follows:
The water pool of the storage unit is determined by the initial water amount of animal excreta from housing, evaporation and additional water that may be added (Fadded water):
By default, the DM content of liquid manure in AMCLIM–MMS is set to 5 %, but it is allowed to vary by a factor of 2, between 2.5 % and 10 %, due to fluctuations in the water pool. Additional water may be added to maintain the DM content within 10 % (fDM.max), as expressed by the following equation:
Covered liquid-manure storage is considered a variation in indoor storage in AMCLIM–MMS. A reduction factor of 0.95 is applied to the NH3 emission from this management system, representing effective mitigation by covering the manure with a lid or covering (Bittman et al., 2014).
Simulations of outdoor storage of liquid manure are similar to those of indoor storage, but the physical and chemical processes are affected by different environmental conditions. The primary difference is the level of turbulence, which is largely related to wind speed and significantly impacts NH3 volatilization. While indoor storage provides a less “windy” environment, external storage exposes liquid manure to the outside environment. Temperature differences between indoor and outdoor storage may be less pronounced. In addition, the water pool of outdoor storage is influenced by rainfall (Frainfall, mm s−1), as expressed by the following equation:
A specific management classified as outdoor storage in AMCLIM–MMS is lagoon systems. Lagoon systems are artificial or natural earthen storage structures that usually provide a largely anaerobic environment for liquid-manure treatment. In this study, a simplified representation of lagoon systems is used, where a constant TAN concentration of 600 µg mL−1 is set for lagoons (Aneja et al., 2001). This simplification is justified as reasonable due to the large amount of water present in lagoon systems, resulting in low TAN concentrations. Therefore, the instantaneous NH3 emission from a lagoon system is expected to be smaller than that directly from livestock excreta, which only disturbs the TAN pool to a limited extent. The process of NH3 volatilization is simulated by the same two-film model as other liquid storage management systems (Sect. S1).
Solid manure has higher DM contents than liquid manure, typically ranging from 30 % to 40 % for pigs and ruminants and up to 50 % to 70 % for poultry manure (Sommer and Hutchings, 2001). With a lower water content, solid-manure storage can facilitate nitrification, providing an additional chemical pathway that depletes the TAN pool, as expressed by the following equation:
The nitrification process in solid manure is similar to that in soils (as presented in Jiang et al., 2024), albeit with some variations in parameters. The details of these calculations can be found in Sect. S4. In solid manure, ammonium can be adsorbed on solid particles, and the manure itself presents an additional barrier to N transport. In AMCLIM–MMS, the partitioning of TAN into different phases in the bulk manure is determined, and the concentrations at the surface are used to calculate NH3 emission. Further information is provided in Sect. S5.
2.4 Land application of livestock manure and ruminant grazing
2.4.1 Application of manure to land
Livestock manure can be used as fertilizer to land either after being stored for a period of time or directly after being removed from animal houses. The land application of manure is simulated by AMCLIM–Land, which employs the four prescribed soil layers (as described in Jiang et al., 2024). The N processes involved in the simulations for manure application are the same as those for synthetic fertilizer applications (Jiang et al., 2024). Specifically, the volatilization processes of NH3 have been described in Sect. 2.2.1 in Jiang et al. (2024). Manure is assumed to be applied only to the soil surface. Modification to allow soil incorporation and deep injection of manure and slurry is possible, but it is not included in the current version of AMCLIM reported here. Stored manure is assumed to be spread on land, and its application is scheduled according to the local crop planting seasons. Alternatively, manure can be applied daily if it is spread soon after being removed from animal houses.
Manure application to land provides sources of N to the soil pools. The soil TAN pool in the top layer can be expressed as follows:
where the application rate ψto land(t) has been shown in Eq. (15). The production of TAN (FTAN) is mainly through the decomposition of organic N. The remaining fluxes are removal processes (FTAN runoff – flux of surface TAN runoff; Fdiffusion – diffusive fluxes; Fleaching – flux of leaching; Fnitrif – nitrification).
Urea in manure is assumed to be fully hydrolysed to TAN during storage upon land application as a simplification, which keeps the soil pH constant. This is true for stored manure and is a reasonable assumption for manure spread daily. Uric acid in poultry manure and organic N are assumed to be retained in the topsoil layer, as these species typically bond with manure and soil particles and are assumed (in AMCLIM) not to move to the underlying layers through diffusion or drainage. These N pools in soils are depleted by hydrolysis or decomposition and surface runoff, which can be expressed as follows:
The runoff of N species (), such as uric acid and organic N, is determined by the following equation:
where rN (mm−1) represents the wash-off factor for N species that is set at 1 % mm−1 (Riddick et al., 2017).
The application of manure, particularly slurry, can affect the soil water content. Misselbrook et al. (2006) reported that 6 mm of pig and cattle slurry infiltrates the soils within an hour following application and causes an increase in the soil moisture content. In AMCLIM–Land, the immediate change in the soil water content after manure application is calculated (Jiang et al., 2024). However, the model does not account for the impact of manure application on soil properties, such as porosity or organic matter content. Additionally, AMCLIM–Land allocates N species in solid manure to the topsoil layer instead of a separate manure layer above the soils.
2.4.2 Ruminant grazing
Grazing practice is an important component of ruminant farming systems. Animals can spend the whole year or part of the year outside (i.e. on pastures or rangelands), corresponding to year-round and seasonal grazing, respectively. Based on the GLEAM livestock data, ruminants are categorized here into grassland and mixed production systems (Seré and Steinfeld, 1996). In AMCLIM–Land, ruminants in the grassland production system are assumed to graze year-round, whereas those in the mixed production system graze seasonally. This assumption has previously been made in the FANv2 model (Vira et al., 2020) and was used here. The NH3 emissions during seasonal grazing are considered to be a counterpart to the housing emissions. The N pools for seasonal grazing can be expressed as follows:
The amount of ruminant excreta deposited on pastures depends on grazing time and is determined from the MMS information provided by the GLEAM model and a temperature condition. Specifically, the fraction of excreta deposited on pastures, fgrazing, is calculated as follows:
where fMMS(pasture) is the fraction of annual total manure deposited on pastures. (°C) is the 10 d running average of daily minimum temperature (calculated for each day of the year), and is the number of days with higher than 10 °C in a year (Pinder et al., 2004). The temperature condition justifies the number of days suitable for grazing in a year, while the MMS statistical data constrain the annual total value of excreted N deposited on pastures. If the values of MMS data (fMMS(pasture)) are smaller than the fraction of suitable days () in a year, ruminants only graze on suitable days (i.e. when is higher than 10 °C). If the MMS value is larger, the AMCLIM model assumes that animals graze throughout the year but spend only a fraction of time outside on pastures daily. This situation counts as seasonal grazing in AMCLIM, even though animals graze year-round, as the grazing system is determined by the production system. Emissions during seasonal grazing can be crucial, particularly if animals are kept outside for a considerable amount of time.
The AMCLIM-Land module includes two schemes for simulating these emissions: the urine patch scheme and the dung pat scheme, as shown in Fig. 3. The urine patch scheme is focused on NH3 emission from urine deposition, while the dung pat scheme considers NH3 from both dung-only and dung–urine mixture situations. These two schemes are analogous to land application of slurry and solid manure, respectively, with the same simulated processes as for the manure application to land.
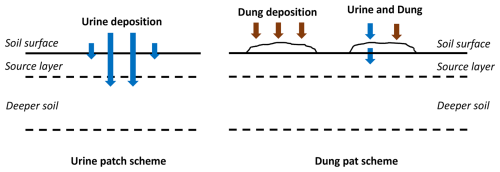
Figure 3Sketch of the urine and the dung patch schemes used in the AMCLIM-Land module for grazing simulations.
Urine can infiltrate into soils relatively quickly and change the water content of the soil surface. Meanwhile, urinary N mainly exists as urea. Hydrolysis of urea in fresh urine results in a soil pH change, which is different from slurry application (where urea is assumed to be completely converted to TAN and not to affect soil pH). Another difference is the vertical soil layering. In the urine patch scheme, only the surface soil layer is modelled, rather than all four soil layers as in simulations for fertilizer applications, in order to reduce computational costs. Furthermore, considering the smaller water volume of ruminant urine compared with slurry application or irrigation, AMCLIM-Land defines a 4 mm source layer in which all simulated processes take place. The thickness of this source layer is based on Móring et al. (2016).
In the dung pat scheme, NH3 is mainly emitted from excreta, rather than the underlying soils, as excreta acts as a substrate to hold the excreted N. An excreta layer is set up above the soil surface in the dung pat scheme, and the underlying soils are not further simulated. All simulated processes in both schemes are the same as those for the topsoil layer of manure applications, and the transport distances for diffusive transport are modified accordingly.
Simulating NH3 emissions from grazing is challenging due to the heterogeneity of grazing fields. It is crucial to determine the area of emitting surfaces with the matched N pools. As animals roam freely and do not urinate and defecate in the same area during every excretion event, fresh excreta does not accumulate on old excreta. In AMCLIM, excreted N from each day is simulated independently and does not accumulate in the common pools. Each day's excreta goes into new pools instead of being added to the previous day's pools. The total NH3 emission from a grazing field can be calculated by the following equation:
where represents the NH3 emission from the area where excreta is deposited on day n. Pools from each day are simulated for 60 d, after which all N pools are assumed to be naturally incorporated into soils and are not simulated further. During the simulation period, input is only from the first day of this 60 d window, and the source area for emissions of each day is a constant value under the assumption that daily excretion rates (urine and dung) remain the same.
2.5 Site-based and global simulations of NH3 emissions from livestock farming
2.5.1 Site simulations of housing NH3 emissions
AMCLIM–Housing was applied at the site scale and used monitored data from experimental farms of Animal Feeding Operations (AFOs) to simulate site-specific NH3 emissions from pig, chicken and dairy cattle houses. The monitored AFOs data were gathered by the US Environmental Protection Agency (EPA) as part of a study of emissions from several types of livestock from 2007 to 2010 (Lim et al., 2010a; Wang et al., 2010). Four houses with slatted floor and pit storage from a pig farm in Indiana (site IN3B) were selected for the simulations, along with two layer houses from a chicken farm in North Carolina (site NC2B) and two free-stall barns in a dairy farm in Indiana (site IN5B), as listed in Table A3. The AFOs datasets provided animal data and daily mean environmental data for the three sites. Animal data included animal numbers, body weight and biomaterial data. Environmental data included indoor and outdoor temperature and relative humidity and the interior ventilation, given as an airflow rate in cubic metres per second (m3 s−1). To keep simulations continuous, missing values in the environmental data due to unavailable measurements were filled by linear interpolation. AMCLIM–Housing used excreted N that was determined from the livestock excreta data (as shown in Table A1) as an input, along with the indoor environmental data. Further information on the measured farms can be found in the US EPA AFOs reports (Lim et al., 2010a; Wang et al., 2010), and a summary of model inputs are presented in Table A4. It is worth noting that the evaluations focused on NH3 emissions from housing, as the processes involved in manure storage are similar to those involved in housing, and there was a limited availability of measurements for NH3 emissions from manure storage. Additionally, the land simulations for TAN application were evaluated against other datasets, as discussed in Jiang et al. (2024).
2.5.2 Global simulations of livestock farming NH3 emissions: input and model setup
Once the AMCLIM model has been applied at the site scale and evaluated against measurement data, the focus is then on applying the model at the global scale. In the present paper, the combined AMCLIM model was applied for 2010 and 2018 to demonstrate full simulations for 2 different years, with activity data and meteorological variables varied between years, so that the inter-annual variability in both emissions and volatilization rates can be analysed. Global simulations of the AMCLIM model were driven by hourly meteorological inputs from the European Centre for Medium-Range Weather Forecasts Reanalysis v5 (ERA5) reanalysis collection (Hersbach et al., 2020), which has been detailed in Jiang et al. (2024). In addition to the input data used by the land module of AMCLIM (AMCLIM–Land), activity data, including livestock and MMS information, are required for simulating livestock farming. The global livestock and MMS data used in AMCLIM are obtained from FAO GLEAM. The global livestock data include information on the geographical distribution of livestock heads, average liveweight and total N excretion rates, which are categorized by production system. The global livestock populations were based on FAOSTAT data for 2010. The geographic distributions were based on the Gridded Livestock of the World (GLW) model, which produced density maps for the main livestock species based on observed densities and explanatory variables such as climatic data, land cover and demographic parameters (Robinson et al., 2014). The reference year of these data is 2010. For simulations for the year 2018, livestock population and N excretion rates were extended by linear interpolation based on the inter-annual variations between 2005 and 2015 suggested by Lu and Tian (2017). The MMS data that determine the fraction of a manure management system are assumed to be constant through the year. Excretion rates of each livestock type are derived from the difference between nitrogen intake and retention based on the GLEAM approach. A summary of model input data for global simulations is given in Table A4. More information on the properties and characteristics of livestock excreta, including urinary N concentrations, faecal N content, dry matter content and pH, is presented in Table A1 in Appendix.
For pig farming, three production systems are used in the global simulations: industrial, intermediate and backyard. For poultry, only chickens are included, which account for over 95 % of poultry by number based on the FAO (FAOSTAT) data for 2010. Chickens have three production systems: broilers, layers and backyard chickens. Ruminants have two production systems: grassland and mixed production systems, except for feedlot cattle, which are treated as a specialized production system: feedlot. In feedlots, cattle are fed with a specialized diet to stimulate weight gain. According to FAO (2018), they are normally kept in concentrated areas to facilitate the fattening processes with high stocking densities. The characteristics and housing features of livestock production systems can be found in GLEAM. The MMS data provide the geographical distributions of the MMS use fractions, which differs between livestock sectors and production systems for pigs and poultry. As described in previous sections, these MMSs are regrouped into the four divisions used in AMCLIM–MMS. More details are available in Table A2 in the Appendix. Land application of manure is assumed to take place throughout the spring and winter planting seasons. All stored manure is applied on fields without explicitly simulating vegetation cover. Section S6 gives detailed global setups for each practice.
To estimate the environmental conditions in livestock houses, empirical relationships between the outdoor temperature and indoor environments, including temperature and ventilation, were developed based on data from the AFOs and theoretical parameterizations of indoor conditions by Gyldenkærne (2005). Equations that present the relationships between indoor temperature, ventilation and outdoor temperature for different housing systems are given in Sect. S6.1. The relative humidity (RH) of indoor environments is assumed to be equivalent to outdoor RH.
Global simulations were performed to estimate NH3 emissions from livestock farming for the years 2010 and 2018, and they had a consistent setup compared to simulations for synthetic fertilizer use, as described by Jiang et al. (2024). AMCLIM was applied using a longitude–latitude grid at a resolution of 0.5° × 0.5°. All model inputs were regridded to the model resolution if necessary. The simulations were performed at an hourly time step, and the prognostic variables at each time step were solved by the Euler method in the model.
2.6 Update of the AMCLIM-Poultry model
Jiang et al. (2021) previously described the development of the AMCLIM-Poultry model (“poultry model” for short in the following text), which provided a starting point and a pilot study that used a process-based model to simulate NH3 emissions from global chicken farming. The poultry model has been incorporated into the full AMCLIM model as a component unit, and several processes have been improved. Major advances in the current AMCLIM model (for simulating poultry farming) compared with the poultry model include the following:
-
The adsorption of TAN on manure particles is included in the current AMCLIM using a linear equation (Sect. S3.2) that describes the equilibrium between aqueous TAN and solid exchangeable TAN.
-
The initial water content of the excreta is considered, rather than assuming an immediate equilibrium moisture content of the excreta.
-
Other organic forms of N in the excreta are included in addition to uric acid.
-
A separate manure management stage is included by operating the AMCLIM–MMS. Litter management is distinguished from other management.
-
Housing of backyard chickens and subsequent manure management replace the original “manure left on land” scenario, according to the characteristics of the production system and the corresponding MMS information (Table A1).
-
The simulations for housing were operated in the updated AMCLIM model at an hourly time step, instead of the daily time step of the original poultry model.
-
Land application of manure is simulated by the land module of AMCLIM, which includes more soil processes and N pathways and employs a four-layer soil profile, as compared with the simpler land application scheme in the poultry model.
-
Nitrogen application rates are derived from recommended or reference manure application rates (Sect. S6.2).
Other smaller changes in the AMCLIM model include the following:
-
The new resistance scheme in the poultry houses consists of a resistance for gas transfer and a litter resistance, rather than using a single constant housing resistance in the poultry model. The newly parameterized gas transfer resistance is dependent on temperature and ventilation inside the house, while the litter resistance is a constant value used the same inversion method as in the previous poultry model (see Sect. S3.2).
-
Manure is no longer only applied to the six prescribed crops based on expert judgement. Instead, manure is assumed to be applied to land depending on a generalized crop calendar which is derived from major crops (see Sect. S6.2).
3.1 NH3 emissions from individual animal houses
The focus of results presented here for the site simulations is the pig houses and dairy barns. Results of layer chicken house simulations are provided in Sect. S7, to avoid repeated content that has previously been presented by Jiang et al. (2021), along with a summary update compared with the prior publication. These site simulations were conducted to evaluate the model performance prior to conducting the global simulations.
3.1.1 Pig houses with slat and pit storage
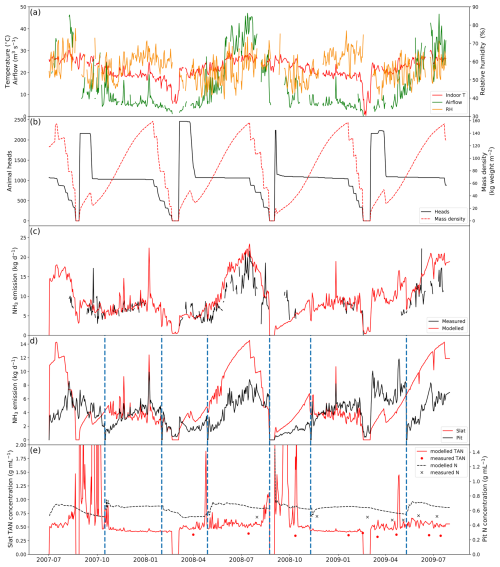
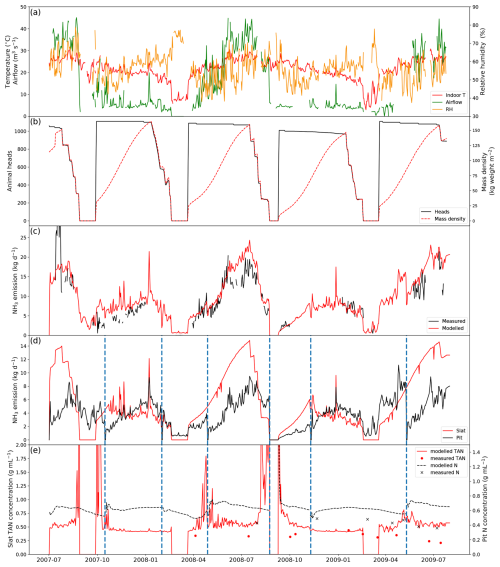
Figure 4 shows the results of simulated NH3 emissions from a pig house in Indiana, USA, with slatted floor and pit storage, alongside comparisons with measurements, stocking data and the indoor environments (simulations for other similarly managed houses are shown in Figs. A1–A3). The simulated period is 2 years from 1 July 2007 to 31 July 2009. Gaps shown in the figure represent unavailable measurements, while the model was kept running to produce a continuous output. The indoor temperature of the pig house ranged between 20 and 30 °C, showing moderate daily and seasonal variations, with a higher temperature in summer than in winter. There were two obvious temperature drops in March 2008 and March 2009 due to the emptying of pigs from the house, as illustrated in Fig. 4b. This also led to low TAN concentration values on slats during the simulation periods. In contrast, the airflow rate inside the house shows significant seasonal variabilities, with higher ventilation occurring in summer (to keep animals cool) and lower ventilation in winter (to keep animals warm). The RH exhibits strong daily variations, ranging from 40 % to 80 %.
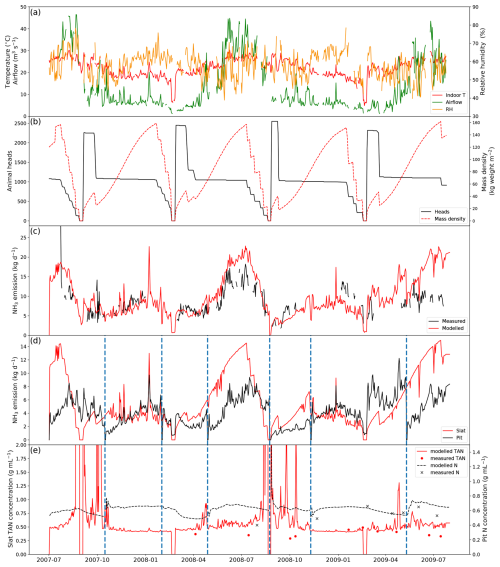
Figure 4Site simulations of House 1 at a pig farm at site IN3B, Carroll County, Indiana, from 1 July 2007 to 31 July 2009. (a) Measured daily mean indoor temperature, airflow rate and relative humidity of the house. (b) Animal heads and mass density of the house. (c) Comparison between the modelled NH3 emissions and calculated NH3 emissions from measured indoor concentrations. (d) Modelled NH3 emissions from the slats and the pit. (e) Comparison between the measured and modelled TAN concentration of the slats and between the measured and modelled N concentration of the pit. Vertical dashed blue lines refer to excreta removal from the pit. See Figs. A1–A3 for the results from other pig houses.
There were several growth cycles of pigs on this farm during the simulated period (Fig. 4b). Over 2000 weaner pigs started in the house, and half of the pigs were moved to other houses after 3–4 weeks once the pigs gained sufficient weight. As a result, the house had twice as many pigs at the beginning of each growth cycle. Approximately 1000–1200 pigs were kept in the house during the subsequent fattening stage.
Measured daily NH3 emissions from the pig house generally increased as ventilation increased. High emissions occurred mostly in summer, with the highest daily values of over 25 kg NH3 d−1 in July 2007. AMCLIM–Housing is able to reproduce the overall trend in the measured NH3 emissions in the first year from July 2007 to July 2008. However, it underestimates the winter emissions (January 2009) by 30 %, which might be caused by overestimated resistances because the simulated TAN concentrations were comparable to the measurements (Fig. 4e). AMCLIM overestimates the summer emissions (June 2009 and July 2009) by a factor of 2 (Fig. 4c), corresponding to higher simulated TAN and total N concentrations for the slatted floor and the pit than those of measurements (Fig. 4e). This is possibly due to underestimations of indoor evaporation in AMCLIM. The average modelled daily NH3 emission value is 10.4 kg d−1 (when measurements are available; 9.9 kg d−1 for the entire simulation period), compared with 8.8 kg d−1 recorded by the measurements. According to AMCLIM–Housing, 42 % of this animal house's total excreted N volatilizes as NH3. The slats and the pit are estimated to contribute 57 % and 43 % of the total emissions, with the average daily emissions being 5.7 and 4.2 kg d−1, respectively. As shown in Fig. 4d, the amount of simulated NH3 emission originating from the slats is typically higher than that from the pits, especially in summer when the ventilation is high. Modelled slat NH3 emissions increase periodically throughout the simulated period, which is closely associated with the animal mass in the house. The dashed blue lines in Fig. 4d and e show when the pit was cleaned. Modelled TAN concentrations on the slats are compared with the measurements as well as with the N concentrations in the pit, with reasonably close agreement being found between the modelled and measured values (Fig. 4e). For the other animal houses assessed, 41 %–42 % of the excreted N was estimated to be emitted as NH3 (Figs. A1–A3).
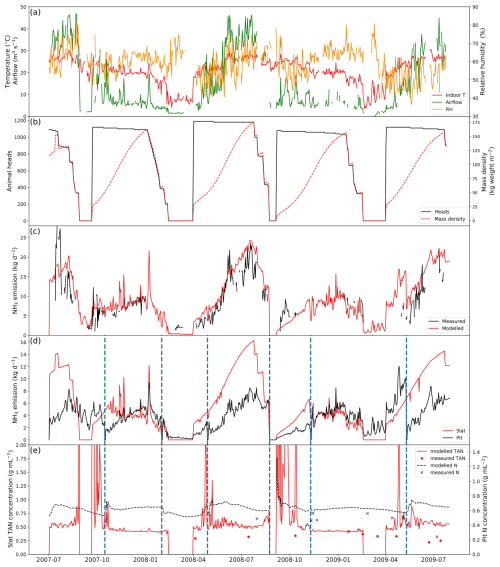
3.1.2 Dairy barns
AMCLIM–Housing was applied to simulate NH3 emissions from two free-stall dairy barns in Indiana, USA. Measurements at these farms were available from 2 years of monitoring. Each barn contained around 1600 Holstein cows. The barns had exhaust fans to facilitate ventilation, and scrapers were used to clean the barn floors and remove manure. More information about the farms can be found in Lim et al. (2010a, b). In the simulations, the cleaning events were assumed to take place every day to represent manure removal by scrapers (Eqs. 6–8). As a result, the N pools and manure pool were reset on a daily basis. Manure removed from barns was not simulated further.
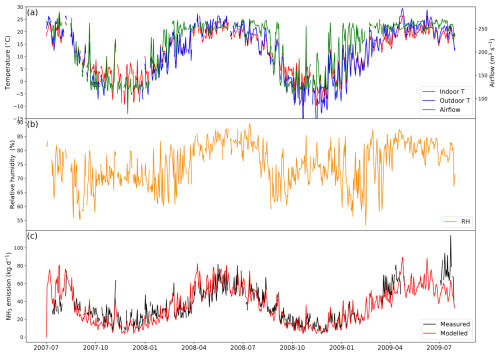
The simulated period is from 1 July 2007 to 31 July 2009, as shown in Fig. 5 (simulations for other similarly managed barns are shown in Fig. A4). The daily average temperature inside the barn is very close to the outdoor temperature, ranging from −10 to 25 °C (Fig. 5a). Strong seasonal variations are found in ventilation, with higher ventilation in summer and lower ventilation in winter, while inside temperature exhibits the same trend. The relative humidity also shows strong daily variations (higher at night), with the highest RH being over 85 % and lowest values being below 55 %.
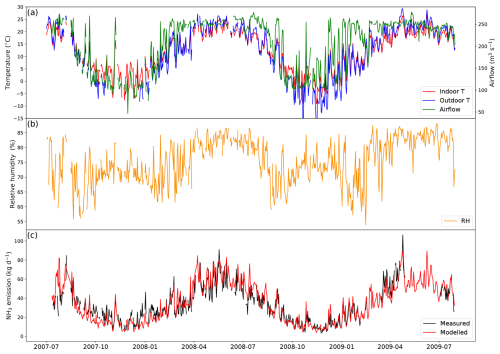
Figure 5Site simulations of Barn 1 at a dairy farm at site IN5B, Jasper, Indiana, from 1 July 2007 to 31 July 2009. (a) Measured daily mean indoor temperature, indoor airflow rate of the barn and outdoor temperature. (b) Measured daily mean relative humidity of the barn. (c) Comparison between the modelled NH3 emissions and calculated NH3 emissions from measured indoor concentrations. See Fig. A4 in the Appendix for the results from the other dairy house.
Overall, AMCLIM–Housing reproduces the NH3 emissions well and captures the daily and seasonal variations. The average modelled NH3 emission from the dairy barn is 32.4 kg d−1 (when measurements are available; 35.2 kg d−1 for the entire simulation), compared with 32.5 kg d−1 reported by the measurements. As shown in Fig. 5, high NH3 emissions occur not only in summer but also in spring, especially in 2009, resulting from high temperature and high ventilation. Meanwhile, emissions decrease in winter when both temperature and ventilation are low. The highest emission is over 100 kg d−1 in April 2009, while the lowest emission is less than 10 kg d−1 on winter days. According to the model for this farm, 15 % of excreted N from dairy is lost due to NH3 emissions for both simulated barns. Overall, the lower volatilization rate for the Indiana cattle houses compared with the North Carolina pig houses (42 %) can be attributed in the model to a combination of a (a) cooler temperatures and (b) scraped floor (removing manure to a separate store).
3.1.3 Sensitivity tests for model parameters of AMCLIM–Housing
Sensitivity tests were conducted to examine the effects of changes in model parameters on the simulated NH3 emission from animal housing, including results for the pig and dairy simulations (described above) and for the chicken house simulations (described in Sect. S7). Nine model parameters with varying ranges were selected for the sensitivity analysis, based on expert judgement, and the corresponding percentage changes in the NH3 emissions are highlighted in Table 1. The estimated pH of excreta used in AMCLIM is identified to be the most important parameter that has significant impacts on the NH3 emissions, especially for the dairy simulations. Varying the evaporation of water in animal houses (Fevap) by a factor of 2 only results in very small changes in emissions compared with other parameters. Moreover, changes in NH3 emissions from layer chicken housing are almost negligible when varying the indoor NH3 concentration by a factor of 2 or setting it to a constant value of 0, which demonstrates the feasibility of neglecting the indoor NH3 concentration in global simulations for chicken housing. The same assumption was also applied to simulations for other livestock.
Table 1Percentage changes in NH3 emissions from pig/dairy and layer housing in the sensitivity tests for the parameters in AMCLIM.
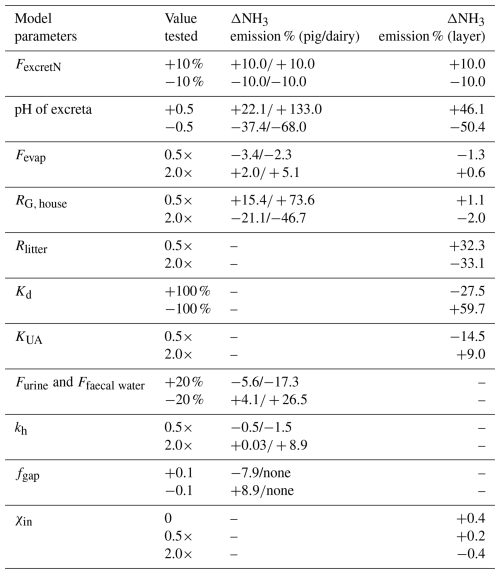
Model parameters in the table are as follows: FexcretN – the total N excretion rate from the livestock; Fevap – evaporation flux of water; RG, house – resistance for NH3 volatilization in the house; Rlitter – poultry litter resistance; Kd – adsorption coefficient of TAN on excreta solids; KUA – uric acid hydrolysis rate; Furine – water from urination; Ffaecal water – water in faeces; kh – urea hydrolysis constant; fgap – gap space of the slats; χin – indoor concentration of NH3.
The NH3 emissions from the housing of all livestock change by the same extent as the changes in N excretion rates (FexcretN). The impact of the housing resistance (Rg, house) on NH3 volatilization in the animal houses is different: NH3 from layer chicken housing is much less influenced by the housing resistance than pig and dairy housing. Litter resistance (Rlitter) plays a more dominant role in affecting the emission than housing resistance for layer chickens as well as for cattle and pigs. The partitioning coefficient for TAN adsorption on excreta solids (Kd) is also important for NH3 emission from layer chicken housing. Excluding the adsorption leads to a 60 % increase in NH3 emissions, while doubling the adsorption results in nearly 30 % less NH3. Although ammonia emissions from chicken excreta are known to be dependent on the uric acid hydrolysis rate, doubling the uric acid hydrolysis rate (KUA) only resulted in NH3 emissions increasing by 9 %, while the emissions decreased by 15 % if the hydrolysis rate was halved. This indicates that much of the uric acid was ultimately hydrolyzed, so that the hydrolysis rate mainly affected the time course of emissions, rather than the total magnitude of annual emissions.
For pig housing, varying the excreta water (Furine and Ffaecal water) by 20 % results in around 5 % changes in NH3 emissions. Doubling or halving the urea hydrolysis constant (kh; details given in Sect. S2) has almost no impact on the NH3 emission. Rapid urea hydrolysis in pig slurry indicates that, even with the sensitivity tests, almost all excreted urea is hydrolyzed to ammonia in these AMCLIM simulations. Increasing the gap space of the slatted floor (fgap) from 0.2 to 0.3 of the house leads NH3 emissions to decline by 8 %, while the NH3 emission increases by 9 % when decreasing the gap space to 0.1. Aarnink et al. (1997) found that more open space on the slatted floor significantly reduced NH3 emissions from the slats, which could explain the decline in total NH3 emissions from the simulated pig houses.
3.2 Global simulations for livestock housing, manure management and land application of manure
In the following sections, emissions are presented in the order of livestock housing, manure management and application of manure, while emissions from grazing are considered in Sect. 3.3. As in the previous sections, pigs (Sect. 3.2.1) and poultry (Sect. 3.2.2) are presented, as representative systems dominated by all-year animal housing. Following this, ruminants, including cattle (Sect. 3.2.3) and sheep and goats (Sect. 3.2.4), are presented, as systems which are complicated by the widespread practice of partial-year housing.
3.2.1 Pig NH3 emissions and volatilization rates
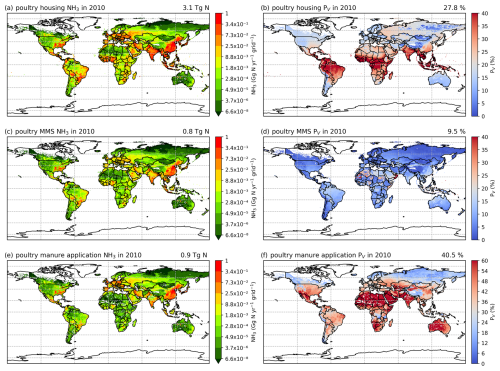
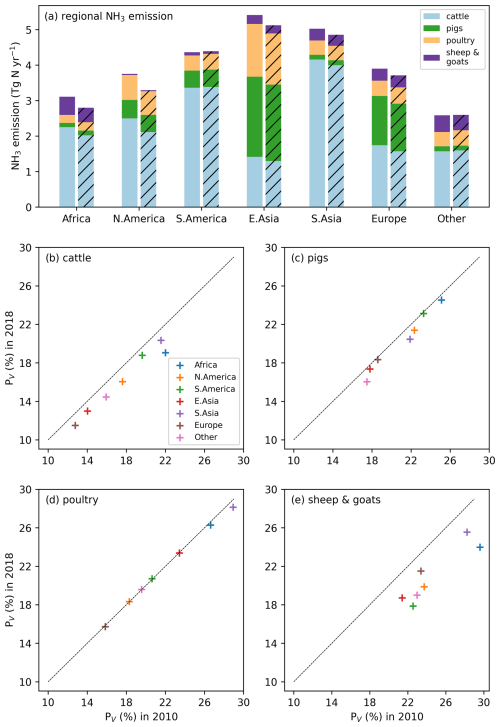
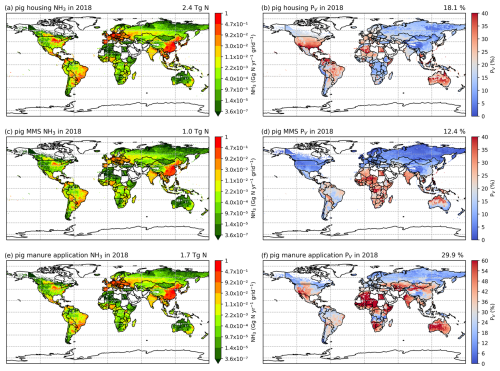
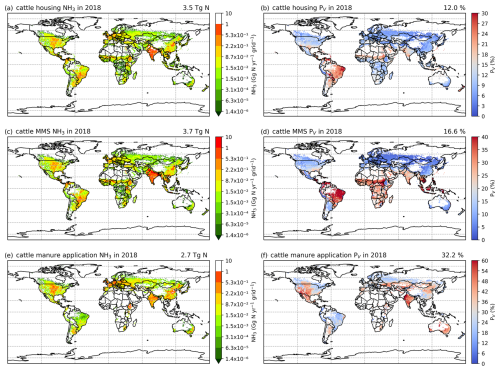
Figures 6 and A5 show the geographical distributions of NH3 emissions from pig agriculture and the volatilization rates for 2010 and 2018. For housing, the volatilization rates (PV) are expressed as a percentage of total N excreted by livestock in the animal houses. For manure management and application to land, the volatilization rates are expressed as a percentage of the total remaining N from the previous stage that is volatilized as NH3. For pig housing and manure management, the spatial distributions of both emissions and volatilization rates are similar for both years. The highest volatilization rates of pig housing (over 35 %) are found in Australia, the USA, Thailand, Malaysia, northern Africa and northern South America. European countries and Brazil also show relatively high volatilization rates of between 20 % and 30 %, while the rest of the world show low to moderate volatilization, ranging from 5 % to 20 %. China, with the highest pig housing emissions, generally has low simulated PV rates of around 10 %. In contrast, Australia shows high PV rates but low emissions. For manure management, manure N volatilization as NH3 can be over 30 % in India, northwestern Australia, Southeast Asia, Africa and several countries in South America, while other regions typically have volatilization rates of less than 20 % (Fig. 6d).
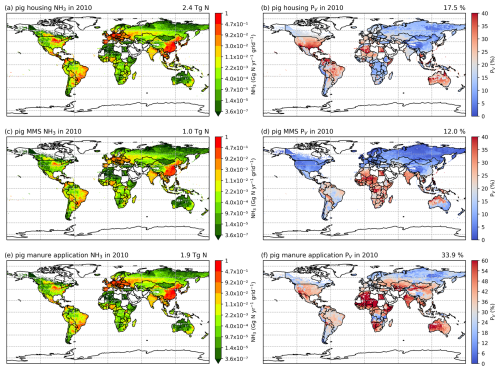
Figure 6AMCLIM pig simulations for the year 2010: NH3 emissions (a, c, e) and percentage volatilization rates (PV) (b, d, f), for housing, MMS and manure application, respectively. Global total NH3 emissions and global average PV values for each activity are shown in the top right-hand corner of the maps. The resolution is 0.5° × 0.5°. White areas indicate zero activity data.
As shown in Fig. 6e, in 2010, high total simulated emissions resulting from pig manure application to land mostly occur in China and Europe. Conversely, high volatilization rates are found in several places across the globe (Fig. 6f): the highest volatilization rates, which exceeded 50 %, can be seen in northern and southern Africa, India, and western Australia. China, Southeast Asia, Europe, the USA and South America showed slightly lower volatilization rates, although these values were also higher than 30 % (Fig. 6f). Only certain countries in Africa, Canada, Scandinavia, and northern and eastern Russia exhibit lower PV rates (less than 30 %). The volatilization rates (PV) in 2018 are sometimes lower than 2010 in several regions, such as southern Russia and the western USA (Fig. A5). The fact that several national boundaries can be seen in the PV maps (Fig. 6b, d, f) shows that these parameter values are not only affected by changes in environmental conditions (which do not generally change suddenly at national boundaries) but also by input datasets that are linked to national conditions in other model inputs. This especially concerns differences in assumed animal liveweights, housing practice and manure management as available from the GLEAM model database, which incorporates national estimates.
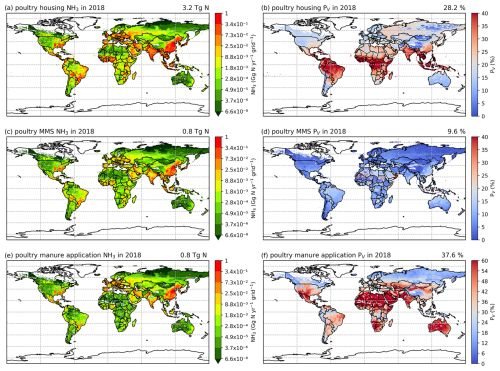
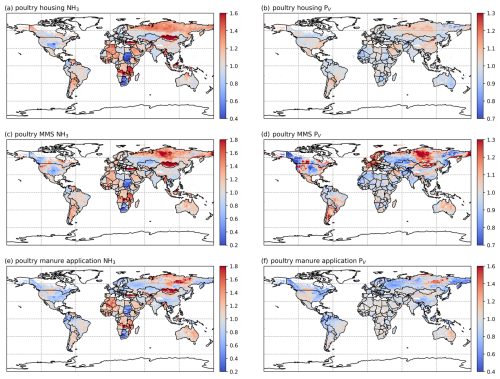
3.2.2 Poultry (chicken) NH3 emissions and volatilization rates
As shown in Figs. 7 and A6, housing and manure application show much higher simulated volatilization rates than manure management. For housing, the highest NH3 volatilization rates of around 40 % are found in tropical regions along the Equator, such as northern South America, central Africa and Southeast Asia. Meanwhile, India and southeast China also show high PV rates of over 30 %. Northern Africa, the Middle East and western Russia have moderate PV values, ranging between 20 % and 30 %, while the other parts of the world have PV rates of less than 20 %. In contrast, volatilization rates for poultry manure management are generally lower than 20 % across the globe, with larger values occasionally occurring in Southeast Asia, Africa and the Middle East. For manure application to land, the highest volatilization rates can exceed 50 %, which can be seen in India, Australia, Mexico, part of the USA, northern and southern Africa, and the Middle East in both 2010 and 2018 (Figs. 7f and A6f). China, Europe and South America also show high volatilization rates of over 30 %. Volatilization rates are generally lower for the year 2018 than for the year 2010, with a clear difference seen in Southeast Asia. As the current model version has updated processes for simulating NH3 emissions from poultry agriculture, a comparison of the results between the current model version, as described in this section, and the previous model version, by Jiang et al. (2021), is discussed in Sect. 4.5.
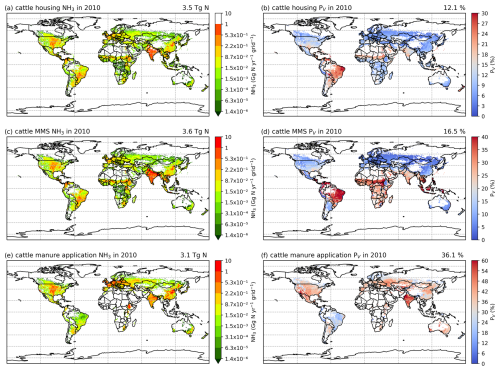
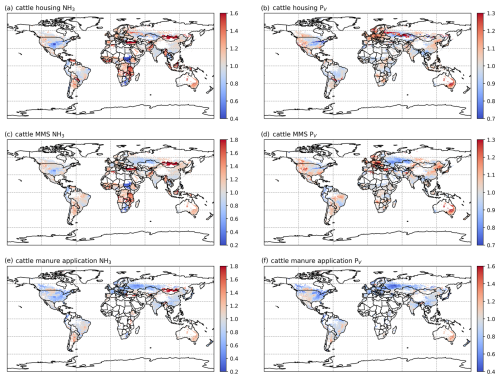
3.2.3 Cattle NH3 emissions and volatilization rates
The geographical distributions of total simulated cattle NH3 emissions and the volatilization rates are shown in Figs. 8 and A7, with no clear difference found for housing and manure management between the 2 years. The volatilization rates for each activity show different patterns. For housing (Figs. 8b and A7b), countries in South America, such as Bolivia, Brazil, Paraguay and Venezuela, and Aotearoa / New Zealand show the highest volatilization rates of over 25 %. Part of India and several Sahel countries show moderate volatilization rates of 15 %–20 %, while the other regions in the world generally have volatilization rates of less than 10 %. For the volatilization rates of manure management (Figs. 8d and A7), the highest rates (of more than 35 %) are found in countries in northern South America (Bolivia, Brazil, Paraguay and Venezuela), the Sahel and Southeast Asia. India, Pakistan, and central and southern Africa also show high volatilization rates of over 20 %. China, the USA and Europe have lower volatilization rates than the regions mentioned above, with typically less than 10 % of N lost through NH3 emissions. By comparison, the percentage volatilization rates are high across most of the regions of the globe (> 36 %), with India having particularly high rates of nearly 60 % (Fig. 8f). Only Brazil, northern Europe and Southeast Asia show lower rates of less than 30 %. Compared with 2010, Russia, the eastern USA and Europe show lower volatilization rates, while volatilization rates remain high in Australia, India, the North China Plain (NCP) and the western USA. Emissions of NH3 related to manure application across Africa are estimated to be small (see Fig. 8e). This is mainly due to very little manure being applied to crop fields in these regions. In AMCLIM, only stored manure is subsequently applied to land as fertilizer (as explained in Sect. 2.3.1), while the majority of cattle excreta in Africa is estimated to be left outside without management according to GLEAM data. Manure left on land without further management is assumed to decompose and be naturally incorporated into soils.
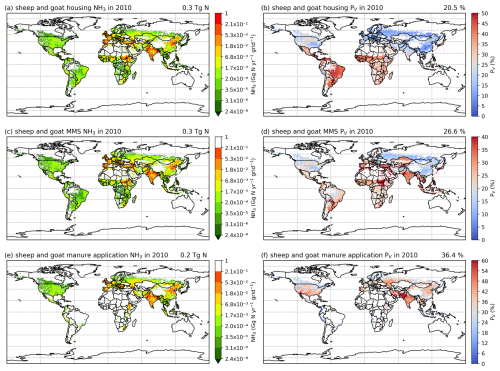
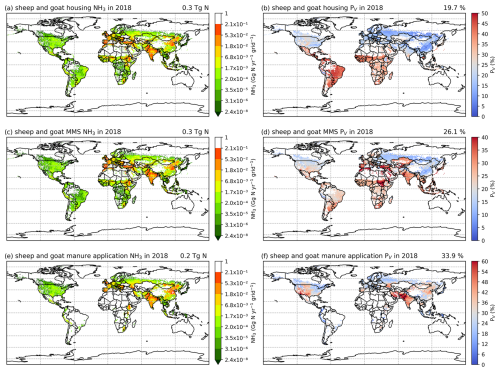
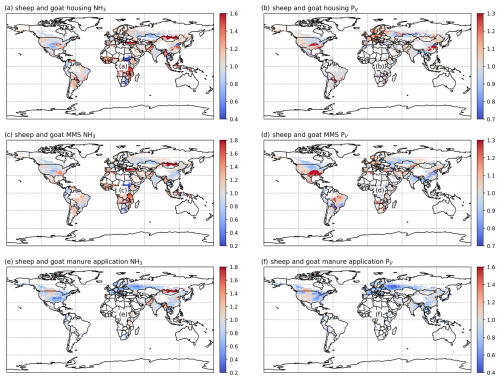
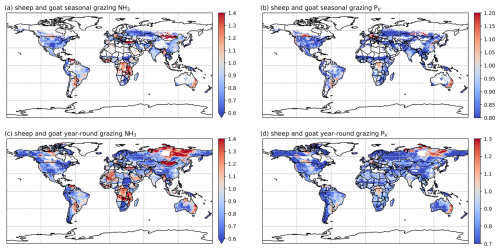
3.2.4 Sheep and goat NH3 emissions and volatilization rates
As shown in Figs. 9 and A8, India, the NCP and Europe typically show higher simulated total NH3 emissions resulting from sheep and goat farming than other regions in the world. However, regions with high emissions are not always consistent with regions with high percentage volatilization rates. For example, the highest simulated housing volatilization rates are found in Africa, South Africa and southern North America, while Asia and Europe generally have lower volatilization rates of less than 20 % (Fig. 9b). Overall, the PV rates for manure management are higher than housing, with large PV found in Africa, Central and South Asia, Europe, South America and Southeast Asia (Fig. 9b and d). For manure application, the corresponding emissions are lower than emissions from housing and manure management, but the volatilization rates are higher. High emissions can mostly be seen in India, the NCP, Pakistan and Europe. High volatilization rates for MMSs are found across all of the regions with emissions, while high volatilization rates occur only in the NCP, Spain, the western USA and South Asia.
3.3 NH3 emissions from ruminant grazing and volatilization rates
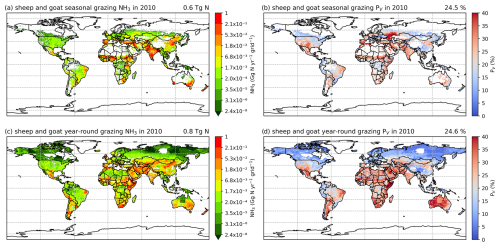
3.3.1 Seasonal grazing and year-round grazing
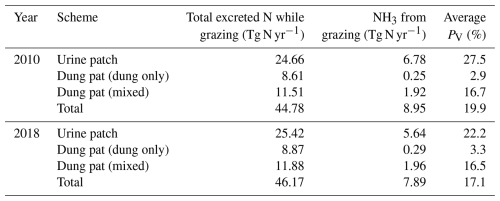
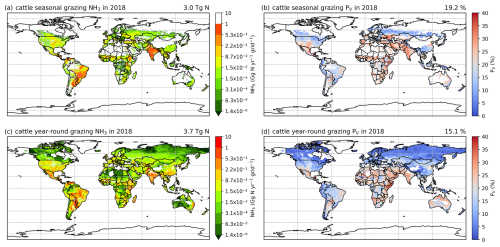
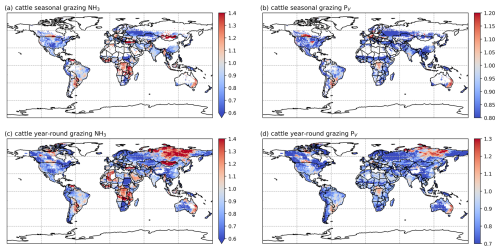
The simulated NH3 emissions from ruminant grazing comprise two parts: emissions from seasonal grazing and year-round grazing. For the seasonal grazing, N excreted by the mixed-production-system cattle is estimated to be 15.3 Tg N yr−1 for 2010 and 15.8 Tg N yr−1 for 2018, accounting for around 35 % of total excreted N, with the remaining 65 % of N being excreted in animal houses. Overall, 21 % and 19 % of the N excreted during grazing is volatilized as NH3 in 2010 and 2018, respectively. As shown in Figs. 10 and A9, high total emissions are found in India, Pakistan and South America, and high percentage volatilization rates are found in France, Mexico, Spain, the southern USA, Africa, South Asia and the Middle East. Beef cattle are the largest emitters, contributing over 50 % of the estimated emissions, whereas dairy cattle and buffaloes are responsible for 30 % and 20 % of emissions, respectively. Buffaloes have the highest percentage volatilization rates, of more than 25 %, reflecting their predominant location in tropical climates, while beef and dairy cattle generally have lower volatilization rates of less than 20 %.
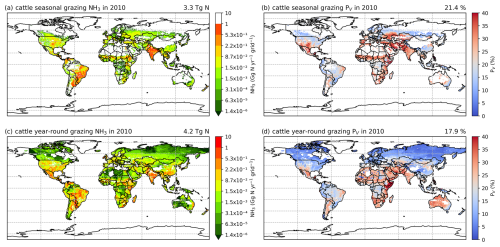
Figure 10Simulated (a) annual global NH3 emissions (Gg N yr−1) from cattle seasonal grazing in 2010. (b) Percentage of excreted N from cattle while grazing seasonally that volatilizes (PV) as NH3 in 2010. (c) Annual global NH3 emissions (Gg N yr−1) from cattle year-round grazing in 2010. (d) Percentage of excreted N from cattle while grazing year-round that volatilizes (PV) as NH3 in 2010. The resolution is 0.5° × 0.5°.
For year-round grazing, total excreted N from grassland-production-system cattle is estimated to be 23.8 Tg N yr−1 for 2010 and 24.4 Tg N yr−1 for 2018, with 18 % and 15 % of excreted N being lost through NH3 emissions in each simulated year, respectively. The overall estimated percentage volatilization rate for year-round grazing of cattle is lower than that for seasonal grazing. Countries and regions with high seasonal grazing emissions also have high emissions from year-round grazing (e.g. Argentina, Brazil, India and Pakistan). Moreover, high emissions also occur in Mexico and the USA. Compared with seasonal grazing, the percentage volatilization rates of year-round grazing are generally lower. High volatilization rates are found across Africa, South Asia, the Middle East and part of Australia. Again, beef cattle contribute over 50 % of total year-round grazing emissions, which makes them the largest emitter. Dairy cattle contributes 40 % of emissions, while buffaloes result in less than 10 %. All types of cattle exhibit similar volatilization rates, with rates in 2018 being lower than in 2010.
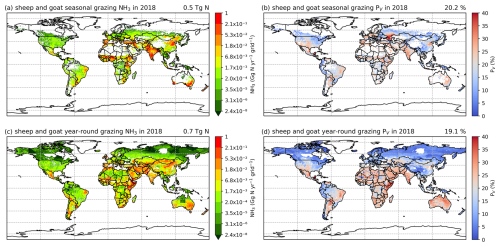
Sheep and goat grazing together resulted in an estimated 1.4 Tg N yr−1 of NH3 emissions in 2010 and 2018, according to simulations using AMCLIM. The mixed production systems contribute 0.6 Tg N yr−1 of emissions, while the grassland production systems contribute 0.8 Tg N yr−1. Contrary to cattle, around 65 % of N in excreta from mixed-production-system sheep and goats is deposited on pastures rather than in houses, with an estimated 25 % and 20 % of excreted N lost through NH3 volatilization in 2010 and 2018, respectively. High emissions occur in India, Iran, the NCP, Pakistan, Spain, Türkiye and several Sahel countries (Fig. 11a and c). The highest volatilization rates are found in southwestern Russia. India, Africa and Europe also have high rates (Figs. 11b and 11d). Sheep contribute over 60 % of total emissions, while goats contribute 40 %. The estimated volatilization rates for both livestock types are similar.
For the grassland production system of sheep and goats, it is estimated that around 25 % and 20 % of excreted N volatilizes as NH3 during year-round grazing in 2010 and 2018, respectively. As shown in Figs. 11 and A10, high emissions are found in southeastern Australia, northern China, eastern Africa, the Middle East and South Asia. High volatilization rates occur in Australia, Mexico, part of the USA, Africa, the Middle East, South Asia and South America (Fig. 11b and d). Sheep are responsible for two-thirds of the estimated emissions, and the volatilization rates for sheep and goats are estimated to be around 20 %. It is notable that year-round grazing of sheep and goats generally results in similar volatilization rates to seasonal grazing, which is different from cattle grazing.
Emissions of NH3 from the different grazing schemes estimated by AMCLIM–Land are summarized in Table 2. In both years, urine patches contribute the highest estimated NH3 emissions and the highest volatilization rates. About 70 %–75 % of NH3 emissions from grazing result from urine patches according to AMCLIM, while the remaining 25 %–30 % of emissions are from dung pats (a combination of dung-only and mixed dung–urine emissions). Within the dung pat scheme, around 3 % of excreted N volatilizes as NH3 from dung itself. By comparison, about 17 % N is lost as NH3 from the mixture of dung and urine.
3.3.2 Comparison of grazing NH3 emissions estimated using AMCLIM with observations
The simulated NH3 volatilization rates from grazing by AMCLIM were compared with measurements, thereby mainly focusing on evaluation against experimental studies that measured NH3 emissions from urine deposition, as NH3 emissions mainly result from urine patches during grazing. Two types of observations were selected for the comparisons: real livestock grazing and urine application. One of the constraints of such studies is that they tend to report insufficient input data in the reported measurements to allow AMCLIM simulations on a detailed site basis (cf. Figs. 4 and 5). Therefore, simulated volatilization rates were extracted from a large number of global experimental studies and compared with the measured percentage volatilization rates derived from these experimental studies, depending on the geographical locations and time of the year.
Figure 12a shows the comparisons between modelled and measured PV for actual livestock grazing. Simulated PV for cattle grazing is comparable to the measurements at sites in the UK (Jarvis et al., 1989a; Ryden et al., 1987), Switzerland (Voglmeier et al., 2018), France (Bell et al., 2017) and Aotearoa / New Zealand (Laubach et al., 2013). The annual mean volatilization rate (% NH3/total excreted N) of grazing in northern Europe estimated by AMCLIM (9.5 %) also agrees with Hutchings et al. (1996) (< 10 %). However, large differences exist between the modelled and measured PV (% NH3/urinary N) for cattle and sheep grazing in the UK (Jarvis et al., 1989b, 1991) as well as between the modelled and measured volatilization rates (% NH3/total excreted N) of cattle grazing in the Netherlands (Bussink, 1992), where AMCLIM largely overestimates the measured volatilization rates. These overestimations might be due to (1) local management practices and (2) the fact that AMCLIM estimates gross emissions rates, excluding possible canopy recapture, which is expected to be more significant in cool, wet climates, such as the United Kingdom and the Netherlands. Bussink (1992) and Jarvis et al. (1989a, b, 1991) measured NH3 loss from grazed land with different levels of synthetic fertilizer inputs that varied between 210 to 550 kg N ha−1. The observed volatilization rates are normally very low (< 5 %), while simulated volatilization rates are much higher (8 % to 22 %). How exactly additional fertilizer affects the NH3 volatilization from livestock excreta on grasslands (e.g. by increasing the N content of urine vs. by direct emission from vegetation) remains unclear.
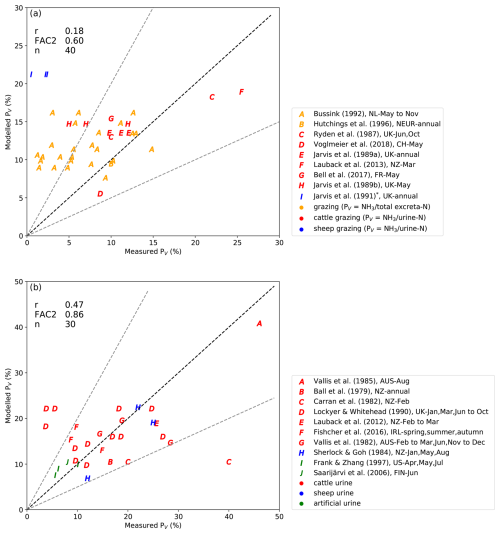
Figure 12Modelled percentage volatilization rates (PV, %) compared with field measurements. Measurement data were from literature that studied real ruminant grazing (a) and ruminant urine application (b). Pearson's correlation coefficient (r), the fraction of values within a factor of 2 (FAC2) and the number of model–measurement comparisons (n) are presented in the top left-hand corner. * In Jarvis et al. (1991), PV of the grazed land with 0 and 420 kg N ha−1 fertilizer input and mixed grass–clover were 0.5 %, 2.2 % and 2.4 %, respectively.
By comparison, there is closer agreement between the volatilization rates estimated by AMCLIM and those measured for urine application than for real animal grazing (Fig. 12a vs. b). Figure 12b shows that the majority of the modelled PV is within a factor of 2 relative to the measured PV (FAC2 = 0.86); the correlation between the model and measurements was 0.47 (r= 0.47). Specifically, PV estimated by AMCLIM is generally consistent with real livestock urine application experiments conducted at sites in Australia (Vallis et al., 1982, 1985), Ireland (Fischer et al., 2016) and Aotearoa / New Zealand (Ball et al., 1979; Laubach et al., 2012; Sherlock and Goh, 1984) as well as two studies using artificial urine in Finland (Saarijärvi et al., 2006) and the USA (Frank and Zhang, 1997). In particular, AMCLIM captures a very high PV measured for cattle urine application in a tropical area in Australia (symbol “A” in Fig. 12b). However, only very limited measurements have been taken in tropical climates, indicating a need for more experiments in hot regions.
It is worth mentioning that, when undertaking a comparison with experiments carried out under dry soil conditions, the volatilization rates of urine application estimated by AMCLIM are either overestimated, for three experiments in the UK summer by Lockyer and Whitehead (1990), or underestimated, for one experiment in Aotearoa / New Zealand summer by Carran et al. (1982). Low PV values measured by Lockyer and Whitehead (1990) in June and July at a UK site show clear differences compared to other measurements of the same study (“D” symbols in Fig. 12b), but the reasons for these differences remained unclear to the original authors, and no clear explanations were provided (Lockyer and Whitehead, 1990). However, as AMCLIM was not applied at each site and was not driven by the same environmental and meteorological variables, the simulated PV is not distinguished between dry or wet soil conditions. Higher PV values in dry soils (soil moisture close to wilting point) than in wet soils (soil moisture close to field capacity) reported by Carran et al. (1982) might be related to (1) the retention of urine in soils and slower drainage and (2) reduced foliar interactions that are expected in wetter situations.
There is less literature investigating NH3 volatilization from dung than urine. In general, the PV of dung was found to vary between 1 % and 5 % in Europe (Fischer et al., 2016; Whitehead, 1990), while Laubach et al. (2013) reported that 11 % of N in dung was lost through NH3 emissions in an experiment in Aotearoa / New Zealand. Meanwhile, it is broadly agreed that NH3 emissions from grazing mainly come from urine deposition, which ranges from 87 % to 96 % based on existing studies (Laubach et al., 2013; Saarijärvi et al., 2006). Simulations using AMCLIM suggest a lower contribution from the urine patch because the mixture of urine and dung in the dung pat scheme was also included in the model. The results from AMCLIM can be considered broadly consistent with experimental studies.
3.4 Nitrogen flows and NH3 emissions of global livestock farming
Figure 13 summarizes the simulated N flows of global livestock farming for the reference year 2010 by AMCLIM, which are allocated to housing, manure management and application to land, with a focus on NH3 emissions. Other simulated nitrogen pathways include surface runoff, nitrification, leaching and diffusion to deeper soils, uptake by plants, and the amount left in soils. As specified in the description of soil processes in AMCLIM (Jiang et al., 2024), denitrification and emission of NO, N2O and N2 are not explicitly included in this study. The flux of “nitrification” simulated in this study can be seen as a sum of both nitrified and denitrified N, with the amount of all relevant species (NO, N2O and N2) being included, as the focus here is on nitrification as a loss pathway of ammonium.
Figure 13Nitrogen budget of global livestock farming estimated by AMCLIM for the year 2010. Activities include housing, manure management systems (MMS), application to land and grazing (for ruminants only). Dark blue arrows are liquid and solid N flows. Red arrows represent gaseous NH3 emissions. All numbers have the unit of Tg N yr−1. The size of the arrows is proportional to the flux.
For pig farming, global total excreted N is estimated at 13.51 Tg N yr−1 in 2010. All excreted N is allocated to housing, which resulted in NH3 emissions of 2.37 Tg N yr−1. A further 2.78 Tg N yr−1 is estimated to be lost because of manure burning (0.50 Tg N yr−1) and unmanaged manure (2.27 Tg N yr−1). The remaining 8.36 Tg N yr−1 undergoes management and leads to 1.01 Tg N yr−1 of NH3 emission. A small part (0.33 Tg N yr−1) is either washed off, nitrified or left in lagoon systems, while 1.32 Tg N yr−1 is left on land without being stored. Subsequently, 5.71 Tg N yr−1 from storage is applied to land, which results in an estimated 1.94 Tg N yr−1 of NH3 emission, 2.87 Tg N yr−1 entering soils and plants, and 0.90 Tg N yr−1 being depleted by other processes (e.g. runoff, nitrification, leaching and diffusion to deep soils). Manure left on land is assumed to be completely incorporated into soils or used by plants and is not further simulated by AMCLIM. Nitrified N was also not further simulated by the model.
Global total excreted N from chickens is estimated at 11.22 Tg N yr−1 in 2010, which resulted in NH3 emissions of 3.10, 0.77 and 0.87 Tg N yr−1 from housing, manure management and application to land, respectively. Only 0.04 Tg N yr−1 is estimated to be burned as fuel, and most manure N (8.07 Tg N yr−1) is managed. During chicken manure management, nitrification and N loss amounts associated with runoff are tiny (0.11 Tg N yr−1). A large fraction of manure N (5.04 Tg N yr−1), which is mainly from deep-litter-system broiler chickens, is left on land rather than being stored. By comparison, 2.15 Tg N yr−1 of stored manure N that is mainly from layer chickens is applied to land. In addition to NH3 emissions, estimated at 0.99 Tg N yr−1 entering soils and plants, the remaining 0.29 Tg N yr−1 is nitrified or lost via runoff, leaching and diffusion to deep soils.
For ruminants, the global total excreted N from cattle, sheep and goats is estimated to be 74.84 Tg N yr−1. About 40 % of N (30.06 Tg N yr−1) is excreted to the housing systems, while 60 % (44.78 Tg N yr−1) is excreted to grazing land. Ruminant housing results in an estimated 3.75 Tg N yr−1 of NH3 emission, while 3.53 Tg N yr−1 of manure N is used as fuel. Manure management results in an estimated 3.89 Tg N yr−1 of NH3 emission, accounting for 17 % of total managed manure N (22.79 Tg N yr−1). During manure management, nitrogen left on land without being stored is estimated at 8.28 Tg N yr−1, while runoff and nitrification together account for 1.62 Tg N yr−1. Nitrogen that is introduced to land consists of two parts: 9.01 Tg N yr−1 from manure storage by land application (17 %) and 44.78 from grazing (83 %), which together result in an estimated 12.20 Tg N yr−1 of NH3 emission (3.25 Tg yr−1 from manure application to land and 8.95 Tg yr−1 from grazing, amounting to PV values of 36 % and 20 %, respectively). Meanwhile, 26.07 Tg N yr−1 enters soils used by plants, and 15.55 Tg N yr−1 of N undergoes other processes (e.g. runoff, nitrification, leaching and diffusion to deep soils).
Overall, NH3 emissions from global livestock farming are estimated to be 29.9 Tg N yr−1, with high NH3 emissions found in China, India, the USA and European countries, accounting for 30 % of the total N from livestock excreta (Fig. 14). Cattle (including buffaloes) are the largest emitter group among livestock, contributing over 60 % of livestock NH3 emissions. Both pigs and poultry result in more than 15 % of livestock NH3 emissions, while sheep and goats are responsible for the remaining 7 %.
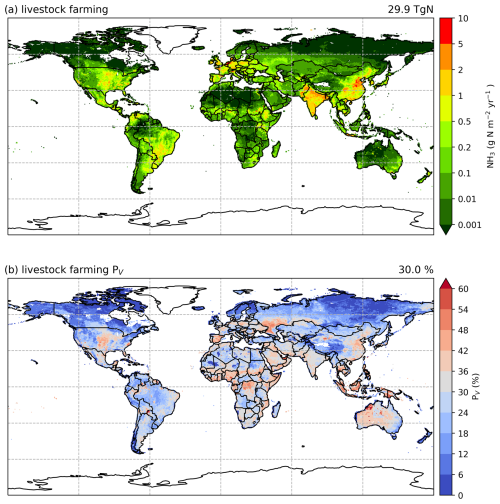
Figure 14Simulated (a) annual global NH3 emissions (gN m−2 yr−1) from livestock farming (including housing, manure management, land application of manure and grazing) in 2010. (b) Percentage of total livestock-excreted N that volatilizes (PV) as NH3 in 2010. The resolution is 0.5° × 0.5°.
As shown in Fig. 15, NH3 emissions and volatilization rates vary across different geographical regions and between the 2 simulated years, i.e. 2010 and 2018. The highest NH3 emissions from livestock agriculture are estimated to occur in East and South Asia. In general, the volatilization rates for livestock are lower in 2018 than in 2010, except for poultry. This is because a large fraction of the poultry, which is comprised of broiler and layer production systems, is assumed (in the model) to be kept in houses with controlled temperature and ventilation, so the PV rates are less impacted by the differences in environmental conditions between years. By comparison, the PV rates from sheep and goats show the largest inter-annual variability among all livestock groups, as sheep and goats typically graze outside and are housed in naturally ventilated barns. As a result, NH3 volatilization from sheep and goats is more dependent on the environmental conditions than poultry. The differences in housing and grazing management also explain why PV rates from pigs showed the second-smallest difference and cattle showed the second-largest difference between the 2 simulated years.
4.1 Contributions to global emissions and evaluation in relation to measurements
Ammonia emissions can occur from various activities in livestock agricultural systems, from housing to subsequent manure storage and the ultimate spreading of manure. The three modules in the AMCLIM model all contribute to simulating a substantial share of total NH3 emissions from livestock farming. Summing for all livestock types, the values are 9.22 Tg N yr−1 from AMCLIM-Housing, 5.67 Tg yr−1 from AMCLIM-MMS and 15.01 Tg yr−1 from AMCLIM-Land, contributing to a global total of 29.9 Tg N yr−1 of NH3 emissions from livestock. The relative shares (31 %, 19 % and 50 %, respectively) indicate the need for all three AMCLIM modules.
AMCLIM has been developed based on prior testing for chicken houses (Jiang et al., 2021), with the model principles building on the earlier simulation approach of the GUANO (Generalisation of Uric Acid Nitrogen emissions) model, a process-based model designed for simulating and predicting NH3 emissions from a source of seabird-derived uric acid, which has been tested in relation to measurements from seabird colonies (Riddick et al., 2017). Extension and further development of AMCLIM–Housing has allowed it to be applied at the site scale to simulate housing emissions and has provided reasonable estimates for pigs, layer hens and dairy cattle, which are all in close agreement with the measurements. This was only possible because of the valuable, detailed and well-documented measurement datasets reported by Lim et al. (2010a) and Wang et al. (2010).
The NH3 emissions from livestock housing are found to be strongly related to the environment of the animal houses: emissions increase with increases in inside temperature and ventilation. Meanwhile, management practices also play an important role in affecting emissions. For example, emissions vary with growing cycles on the pig farm at site IN3B, and the removal of excreta causes a short “stoppage” in NH3 emission (see Fig. 4). Unlike the pigs and layer chickens, the indoor conditions of the dairy barn are closer to the ambient environment, which is generally cooler throughout the year. As a result, the annual average volatilization rates are lower for dairy barns compared with pig and layer chicken houses.
In contrast to the simulations of NH3 emissions from housing, there are many emission datasets for grazing livestock and for urine application to land, but these lack the details needed to allow specific site application of the model, as done for housing. Accordingly, the comparison with measurements in Fig. 12 focuses on a multi-site comparison for a wide range of published datasets globally. While this comparison is useful, it also highlights the great value of publishing carefully documented detailed time-resolved datasets as reported by Lim et al. (2010a) and Wang et al. (2010). Future work should look to acquire further quality-assured measurement datasets for additional testing.
4.2 Comparison of AMCLIM with previous studies and emission factor approaches
Estimated NH3 emissions in this study are generally in line with the analysis by Uwizeye et al. (2020). Global total emissions from livestock agriculture were estimated to be 29.9 Tg N yr−1 according to AMCLIM simulations, while Uwizeye et al. (2020) reported that the global livestock supply chain contributed 26.4 Tg N yr−1. Emissions from animal production related to housing and manure management are higher from AMCLIM (14.9 Tg N yr−1) than those from Uwizeye et al. (2020) (11.6 Tg N yr−1), while estimated NH3 emissions from land application of manure to cropland and grasslands (including grazing) by AMCLIM (15.0 Tg N yr−1) are comparable to the emissions from feed production estimated by Uwizeye et al. (2020) (14.8 Tg N yr−1). Sutton et al. (2013) also reported a similar emission value of 15 Tg N yr−1 NH3 from crops and grasses used in livestock production.
Although AMCLIM is a dynamical process-based model that is not dependent on emission factors (EFs), results of its simulations can be averaged as a basis for comparison with existing EFs from other studies and emission inventories. Livestock-specific EFs for NH3 derived from AMCLIM simulations and comparisons with EFs from the reviewed range of literature reported by Yang et al. (2023) are summarized in Table 3. On a global scale, the EFs derived from AMCLIM are generally comparable to the values from Yang et al. (2023). However, AMCLIM predicted higher EFs for buffaloes and lower EFs for dairy cattle than the literature reviewed by Yang et al. (2023). Although this broad comparability may be considered encouraging, one of the key reasons for developing AMCLIM is to illustrate how climatic variation affects ammonia emission. Such effects are not well treated by average EFs; therefore, it is not appropriate to search for exact agreement, which cannot be achieved by the EF approach.
Table 3Simulated animal NH3 emission factors (EFs) (kg N per head per year) for livestock derived from the global simulations of AMCLIM compared with Yang et al. (2023), who summarize the range of EFs from the literature. The AMCLIM values in the first row are the global mean EFs, while values in parentheses represent the 10th and 90th percentile of the 0.5° × 0.5° resolution values, respectively.
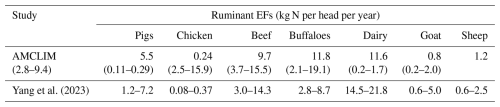
Table 4 compares the EFs (expressed as % of TAN) simulated by AMCLIM with EFs reported by EMEP/EEA (2019) and Sommer et al. (2019). The table summarizes both global mean and European Union mean EFs, with the range of EFs between the 10th and 90th percentile of both spatial scales. As shown in Table 4, estimated EFs for livestock housing and manure application to land from this study are generally comparable to the values from literature and reports. The EFs of manure storage derived from AMCLIM simulation are often lower than those from EMEP/EEA (2019) and Sommer et al. (2019), while the grazing EFs are higher than those from previous studies. As noted in Sect. 3.3.2, the simulated volatilization rates from AMCLIM may be higher than those from other studies, as AMCLIM estimates gross emission without accounting for canopy recapture, which can be significant for grassland contexts, especially in wet climates (Massad et al., 2010; Sutton et al., 2013). For housing, AMCLIM did not differentiate slurry from solid manure for ruminants. The largest difference is from broiler housing, the EF of which is 2 times higher than that reported. Although estimated EFs for manure storage in this study are low, the AMCLIM model showed that slurry storage typically had lower EFs than solid manure, which is consistent with EMEP/EEA (2019). Both this study and EMEP/EEA (2019) agreed that the highest EFs are from manure application among the four practices. However, AMCLIM also takes organic N other than urea into account. The organic N from dung can be a slow but significant source to the TAN pool as a result of mineralization. In all AMCLIM simulations, NH3 emissions volatilized from the TAN pool were not differentiated from urea N or other organic N. In order to include this effect in the comparison, a set of correction factors were applied to the total N to obtain the amount of TAN. It is assumed that the TAN amount is the sum of the urea fraction and half of the organic N fraction (the “available organic nitrogen compound” assumed in the model, which accounts for 50 % of organic nitrogen other than urea; see Sect. S2) of livestock excreta. As a consequence, estimated EFs are more robust for housing and grazing in terms of NH3 lost as percentage of TAN compared with manure storage and application to the field, as the quantities of different N forms in the latter stages are more uncertain and difficult to estimate. On the one hand, it has been argued that the EF method is not ideal for calculating NH3 emissions due to its limitations with respect to excluding the climate-dependence of NH3. On the other hand, the EFs given as the percentage of TAN do not explicitly include the organic N input from manure, which might result in either overestimation or underestimation in NH3 emissions, depending on the activity data used.
Table 4Averaged simulated NH3 EFs expressed as a percentage of TAN for livestock derived from the global simulations of AMCLIM compared with EMEP/EEA (2019) and Sommer et al. (2019). Values in parentheses represent the 10th and 90th percentile of the 0.5° × 0.5° resolution values, respectively.
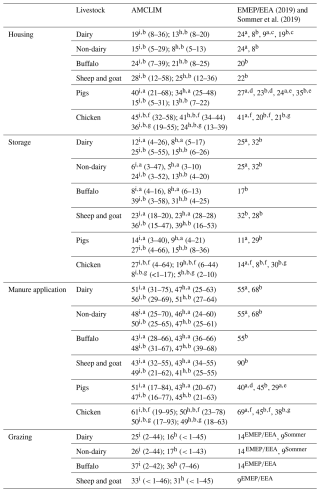
a Slurry. b Solid manure. c Tied housing. d Finishing pigs (8–110 kg). e Sows and piglets (up to 8 kg). f Laying hens. g Broilers. h Global mean EF. i Mean EF for Europe.
4.3 Spatial distribution of simulated ammonia emissions in relation to climate and management
The spatial distributions of both emissions and the percentage volatilization rates simulated by AMCLIM, as shown in Figs. 6–11 and 14, demonstrate substantial variations. High values of total emissions (expressed as kg N per grid per year) primarily coincide with high animal populations in countries or regions with intensive livestock farming, such as China, India, the USA and Europe.
The volatilization rates (expressed as the percentage volatilized, PV) differ across the globe due to a combined effect of environmental conditions and management practices. High simulated volatilization rates of chicken housing are found in the tropical regions along the Equator, indicating that hot and humid conditions tend to cause larger emissions. Among cattle and buffaloes, the overall simulated volatilization rates for buffaloes are higher than other types of cattle. This is because buffaloes are predominantly reared in hot regions such as southern China, South Asia and Southeast Asia compared with other cattle, which are widely distributed across the globe, resulting in higher PV values for buffaloes due to generally hotter conditions. Moreover, the estimated volatilization rates for sheep and goat farming are higher than those of cattle farming, partly due to a higher N concentration in sheep and goat urine compared with cattle. Another reason is that sheep and goats are more “concentrated” in the Middle East and South Asia, where they tend to have higher volatilization rates due to warmer climates. In addition to temperature, soil pH plays an important role in NH3 volatilization. As pointed out by Jiang et al. (2024), simulated high PV values have been found in regions with high soil pH, such as the western USA, Namibia, Mongolia and part of northern China.
Various management practices can lead to very different volatilization rates. For housing, industrial pig husbandry shows higher volatilization compared to intermediate and backyard pig husbandry. This is due to the fact that pigs in an industrial production setting are kept in buildings with heating systems, and excreta is kept longer in the houses because in situ storage is available. Moreover, the pits for manure storage provide an additional emitting surface of NH3. The housing density assumed in AMCLIM is another factor that affects the volatilization rates. The volatilization rates of feedlot cattle housing are the second lowest among ruminants. This is partly because feedlot cattle have the highest stocking density in the model. Increasing the stocking density results in a smaller source area for NH3 emission, which leads to lower emissions.
For manure management, especially in warm climates, manure left on land without much management is identified to result in much higher NH3 emissions than manure that is stored either as liquid or solid manure, leading to higher PV values. Such a practice is common in Africa and some countries in South Asia, like India and Myanmar, and these regions have a hot climate (as reflected in high PV values). Conversely, manure storage under cover greatly reduces NH3 emissions (Bittman et al., 2014). Although the effect of covering stored manure is not the focus of the present study, the process-based nature of AMCLIM would lend itself to a future examination of such effects.
4.4 Comparison of ammonia emissions and volatilization rates in 2010 and 2018
The AMCLIM simulations were calculated for 2 recent years (for which meteorological data were available) in order to illustrate how annual weather differences could influence NH3 emissions globally. Of the 2 years studied, temperatures and water availability conditions changed across the globe. In general, regions with a higher temperature in 2018 than 2010 had less precipitation and drier soil conditions (Fig. A11). As summarized in Table 5, the overall estimated volatilization rates of livestock farming for the year 2010 are found to be very similar to the values for 2018. Specifically, NH3 emissions from housing and manure management for pig and chicken agriculture show small differences between 2010 and 2018, with slightly higher PV values occurring in 2018 compared to 2010 (reflecting warmer conditions in 2018).
Such relatively “stable” PV rates for housing and manure storage are possibly due to the largely controlled indoor environments of animal houses compared with natural conditions (as shown in Figs. A12). Enclosed animal houses have their own regulated temperature inside; moreover, naturally ventilated barns are not as windy as outside, and the floor temperature of these barns is less varied than the air temperature. For manure management, although the global average PV values between the 2 simulated years are similar, the changes in PV are site-specific. As shown in Figs. A12 and A13, the differences in the PV values of the manure management of chickens and pigs between 2010 and 2018 show stronger spatial variations than those of housing (as shown in Figs. A12 and A13), so the two very similar annual volatilization rates could be a result of global averaging of various manure management systems.
Compared with housing and manure management, emissions from land application of manure vary by a larger amount between the 2 years for all livestock groups (Table 5; Figs. A6–A10 and A12–A17). In this case, 2010 shows both higher total emissions and percentage volatilization rates compared with 2018 (reflecting that 2018 was wetter than 2010). Such inter-annual differences in NH3 emissions from manure application to land are found to be consistent with AMCLIM simulations for synthetic fertilizer application, as discussed in Jiang et al. (2024). The relevant processes that govern the NH3 emissions from land application are dependent on naturally varying environmental conditions, while there are more N pathways, such as runoff, drainage and diffusion. As more processes are involved under natural conditions, NH3 emissions may show larger variations. The simulations by Jiang et al. (2024) for synthetic fertilizer application indicate that the lower volatilization rates in 2018 (compared with 2010) can be attributed to higher leaching and diffusive fluxes in 2018 (reflecting the wetter conditions in 2018 compared with 2010), which deplete the soil N and lead to lower NH3 emissions. The different PV values of manure application to land between the 2 years, shown in Table 5 and Figs. A12–A17, may result from the same reason. These differences are evident from the 2010 vs. 2018 comparison for different livestock types, reflecting these differences in source category contributions to the emissions from each livestock type (Fig. 15). Finally, Fig. 15 shows that the largest relative difference between emissions for 2010 and 2018 applies to sheep and goats, with lower PV values in 2018. This is because NH3 emissions from sheep and goats are dominated by grazing; thus, the wetter conditions in 2018 had a larger effect than the warmer temperatures in 2018 (Table 5).
Table 5Annual mean volatilization rates of livestock housing, manure management, land application of manure and grazing in the years 2010 and 2018. Annual mean temperature, soil water content and subsurface percolation flux for locations where NH3 emissions occur and where these three activities took place in 2010 and 2018.
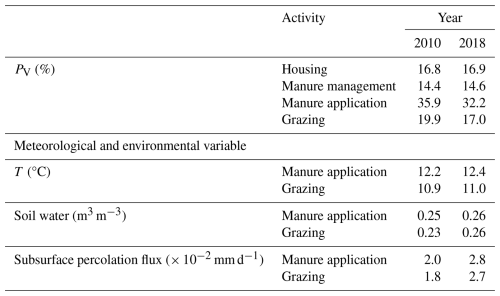
4.5 Global chicken farming: comparison with the previous version of AMCLIM
As described in Sect. 2.6, several further developments are included in the AMCLIM simulations for ammonia emission than in the earlier “poultry model” previously reported by Jiang et al. (2021). With the improvements and modifications, the current AMCLIM model provides very similar estimates of the housing simulations at the site scale (see Sect. S7). This can be explained by the various process parameterizations that have opposite effects. The inclusion of other organic forms of N gradually expands the TAN pool, which leads to more N that is available for NH3 emissions. Conversely, the adsorption of TAN on manure solids and more moisture in the excreta decrease the aqueous TAN concentration, compensating for this effect.
For the global simulations, NH3 emissions from chicken farming are estimated at 4.8 Tg N yr−1 in 2010 by the current AMCLIM simulations, which is about 13 % less than those from the poultry model's estimation of 5.5 Tg N yr−1. The relative contribution to the total emissions shifts from the land application of manure to housing, which is largely due to the fact that emissions from backyard chicken husbandry are counted as housing emissions in the current AMCLIM version, rather than as part of land application emissions as in the earlier poultry model.
Housing emissions from broilers are comparable between AMCLIM and the poultry model, whereas AMCLIM estimates higher housing emissions from layer chickens compared with the earlier model. Lower land application emissions were estimated by the current AMCLIM model, which is partly because (1) less N is applied to land, (2) more N pathways that are included act as competing fluxes to volatilization and could decrease the emission, and (3) the adsorption of TAN on soil solids leads to a lower emission potential.
4.6 Ruminant grazing
Grazing is an additional component of the simulations for ruminant farming compared with pigs and poultry. The estimated NH3 emissions from grazing are 9.0 and 7.9 Tg N yr−1 for 2010 and 2018, respectively, accounting for corresponding amounts of around 19 % and 16 % of excreted N from ruminants while grazing. Emissions related to grazing exhibit the largest annual difference between the 2 simulated years compared with other activities. The total grazing emissions estimated by AMCLIM are lower than the 12 Tg N yr−1 suggested by the FANv2 model (Vira et al., 2020), but the volatilization rates are comparable to the 18 % reported by FANv2. The differences in emissions between AMCLIM and FANv2 are partly due to the different estimates of excreted N on pastures. In general, excreted N on pastures during grazing results in a lower simulated volatilization loss of NH3 in AMCLIM compared with manure application.
For the mixed production system, about 65 % of N in cattle excreta is excreted in animal houses, compared with less than 40 % for sheep and goats, based on the GLEAM MMS data. The volatilization loss of excreted N during the seasonal grazing of cattle is around 20 %, which is similar to the value for sheep and goats. It should be noted that the regional variations in the volatilization rates of year-round sheep and goat grazing are larger than those for seasonal grazing (Fig. 11b, d). By comparison, the regional variations in the year-round grazing of cattle are similar to those for seasonal grazing (Fig. 10b, d). As a result, with the grassland production system (year-round grazing) being more widely spread across the globe, especially in temperate and cold regions, compared with the mixed production system, year-round grazing of cattle shows lower volatilization rates compared with seasonal grazing, while there is not much difference between year-round and seasonal grazing for sheep and goats.
It is evident that the urine patch scheme in the grazing simulations results in much higher NH3 emissions and a higher volatilization rate compared with the dung pat scheme (Fig. 3). Urea in urine deposited on pastures is readily able to hydrolyse to TAN, which can lead to higher emissions than dung due to the slower decomposition of organic forms of N in dung. Existing experimental studies have reported that NH3 loss accounts for 0.5 %–46 % of urinary N, while AMCLIM predicted 5.5 %–41 % (Fig. 12). These differences can be caused by different environmental and meteorological conditions, such as temperature, soil moisture, precipitation and soil texture, between the year when experiments were conducted and the modelled year 2010. It is also still unclear why several experiments showed very low volatilization rates; this was not clearly explained by experimentalists and needs further investigations (see Lockyer and Whitehead, 1990; Jarvis et al., 1991). Overall, estimated volatilization rates by AMCLIM are broadly consistent with measurements (Fig. 12), especially in warm regions such as Australia and Aotearoa / New Zealand, but they are overestimates compared with some measurements from wetter climates, such as in the UK and Netherlands. The differences indicate that the infiltration and drainage (also diffusion) in AMCLIM might not be sufficiently representative. In addition, it should be noted that the present version of AMCLIM does not include a vegetation layer and, therefore, represents gross emissions from the land surface. In cool, wet climates, bi-directional NH3 exchange can occur from grazing land, and the low percentage of ammonia emissions reported by Jarvis et al. (1989a, b) can be considered as net fluxes including this effect. Considering such bi-directional interactions is to be treated as part of the future development of AMCLIM.
In comparison with urine, dung contributes less NH3 emissions, due to the lower volatilization rates from dung. However, the dung–urine scheme implies that urine deposited on dung can also result in considerable NH3 emissions, due to the slow infiltration of urine to the soil underneath, as dung partly retains the liquid. A similar example is the surface application of manure to land, which can cause large NH3 emissions as a percentage of the N applied, highlighting the need for the immediate incorporation of manure to reduce emissions.
4.7 Uncertainty and limitations
Uncertainty in NH3 emissions from livestock farming simulations arises from multiple sources, as illustrated by the sensitivity analysis (Table 1). Here, it was shown that the largest overarching uncertainty in the model (relevant for all modules) is the pH of an emitting surface. In practice, surface pH changes dynamically, also in response to urea and uric acid hydrolysis, as the production of NH3 increases pH (Bittman et al., 2014). Although it is possible to simulate surface pH dynamically, as shown by Móring et al. (2016) for grazing, this is computationally expensive and brings its own uncertainties, make it less appealing for application in global simulations. In the present study, empirical pH values were used based on Chantigny et al. (2004) and Móring et al. (2016). However, in practice, estimating a bulk surface pH of the emitting solution can also be considered as a potential model tuning parameter for simulations in relation to measured NH3 emissions.
In addition to the effect of pH (Table 1), the adsorption of TAN on manure particles can be considered to be a further uncertainty. This is represented by a linear relationship with a constant coefficient that describes the equilibrium, which may influence the calculation of TAN concentrations (see Sect. S3.2). Uncertainty associated with the adsorption scheme mainly exists in solid-manure simulations, such as poultry simulations and solid-manure storage.
For the housing simulations, the relationships used to parameterize the indoor conditions may not be representative, as they are largely derived from farms in the USA. Moreover, ventilation in the naturally ventilated barns can be uncertain, which can influence the rates of the simulated processes. In addition, for pit housing systems, the gap area between floor slats and the width of the slats in animal houses may vary in practice. AMCLIM used a fixed value that assumes a 20 % gap space for the slatted floor. Furthermore, the surface area of the pit for manure storage may not be the same as the floor area above, even though it was assumed to be equivalent to the floor area in AMCLIM.
For manure management simulations, the largest uncertainty comes from the source area. In AMCLIM, the area for NH3 emissions at this stage was assumed to be proportional to the housing area, based on the logic that more area might be required for manure storage for more animals. This is a reasonable assumption, but the ratio is unclear. The determination of the NH3-emitting surface during manure storage can be considered as a major limitation of AMCLIM because it has a significant effect on emissions, but detailed global data on these differences are apparently unavailable.
Only surface broadcasting was simulated for land spreading of manure, which may not reflect reality in countries with policies that require manure to be incorporated into soils, such as the Netherlands and Denmark. Emissions from manure application are expected to be overestimated in these places. In order to incorporate such effects in the global simulations of AMCLIM, an international database would need to be established that provides statistics on the extent to which solid and liquid manure are immediately incorporated, injected or applied using band-spreading (see Bittman et al., 2014; Sutton et al., 2022). In principle, AMCLIM is well suited to treating such effects. Other uncertainties in the land application that also influence the grazing simulations have been discussed in a companion paper (Jiang et al., 2024), including the following: input data for soil characteristics (soil texture, pH and organic matter content), the representation of soil pH dynamic after urea deposition during grazing, and linear relationships used for calculating diffusive and drainage fluxes of N species.
This paper presents the development and application of the AMCLIM model to simulate NH3 emissions from livestock farming, including pigs, poultry (chickens), cattle, sheep and goats. AMCLIM follows the N flow from animal housing and manure management to the ultimate land application of manure, with impacts of environmental factors being included in the model. AMCLIM–Housing includes two housing systems and three housing types, and AMCLIM–MMS includes four major manure management types, which allows the impacts of management practices to be reflected; i.e. simulations for livestock sectors and production systems can be differentiated. AMCLIM has also incorporated substantial updates for simulating poultry farming emissions, with more processes being included compared with the previous version outlined in Jiang et al. (2021).
A major effort has been made to evaluate AMCLIM–Housing against measurements from the US EPA AFOs. The simulated NH3 emissions from pig, layer chicken and dairy cattle housing showed close agreement with measurements. AMCLIM–Housing was able to (1) broadly reproduce the NH3 emissions from two types of animal houses with different processes and settings and (2) roughly replicate the daily variations in NH3 emissions. The other two modules were not specifically tested against measurements because of the similarities in the processes for housing and manure management and the lack of available datasets. By comparison, AMCLIM–Land has been tested elsewhere, and details are given in Jiang et al. (2024). In the case of grazing, the lack of comparably detailed measurement data to the US EPA studies meant that this study focused on a multi-site global comparison with average emissions from studies according to climatic and management differences. While the detailed and multi-site comparisons with measurements are encouraging, there is an ongoing need for detailed reporting of quality-assured NH3 flux measurements with known uncertainties as a basis for further model–measurement comparisons.
Based on simulations using AMCLIM, global livestock farming results in an estimated 29.9 Tg N of NH3 emissions for the reference year 2010. Specifically, cattle are found to the be the largest emitting group, resulting in 17.7 Tg N yr−1 of emissions; pig farming is estimated to contribute 5.3 Tg N yr−1; chicken farming results in 4.8 Tg N yr−1; and sheep and goats combined lead to the remaining estimated 2.4 Tg N yr−1. This indicates that, overall, around 30 % of total excreted N is lost due to NH3 emissions. High emissions from livestock farming are typically found in Brazil, China, India, the USA and Europe, coinciding with regions that have high livestock population numbers. The volatilization rates show strong spatial variations across the globe, with the highest volatilization rates being up to 60 %–70 % of excreted N. In particular, hot regions generally exhibit higher volatilization rates than cold areas with respect to livestock farming. By comparison, moisture conditions can have different effects regarding the specific livestock management; i.e. wet conditions can facilitate uric acid hydrolysis and cause larger NH3 emissions for poultry (chicken) housing. Conversely, drier conditions increase the concentration of urea and TAN for other livestock, and the emission potential can therefore be higher. These findings once again demonstrate that simple EFs maybe not sufficient to reflect real-world conditions and that there is a need to refine current EFs to incorporate the climate dependence of NH3 emissions.
As compared with the reference year of 2010, results of simulations for 2018 show little overall difference in annual volatilization rates for livestock housing (16.8 % for 2010 and 16.9 % for 2018) or manure management (14.4 % for 2010 and 14.6 % for 2018). In contrast, land application of livestock manure shows more inter-annual variability than housing and manure management, with obviously lower volatilization rates in 2018 (32.2 % for 2018 vs. 35.9 % for 2010). This is consistent with differences observed between 2010 and 2018 for simulations of NH3 emissions from synthetic fertilizer (Jiang et al., 2024), reflecting that the year 2018 was a hotter but wetter year than 2010. Such a phenomenon is largely due to the fact that the environment of housing and some manure management practices are controlled and vary less than emissions from land. The estimates from the AMCLIM model emphasize the importance of both environmental factors and local management practices. Compared with the traditional approach of estimating emission factors, AMCLIM provides an appropriate tool to allow process-based estimation of the ways in which climatic and management factors may affect NH3 emissions at site, regional and global scales.
Table A1Biomaterial and characteristic information of livestock excreta as used in AMCLIM.
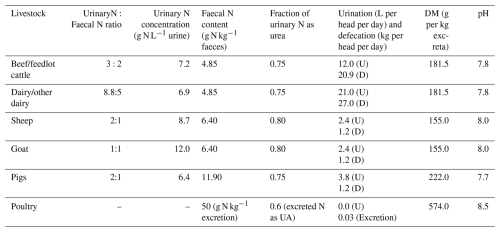
Abbreviations used in the table are as follows: UA is uric acid; U is urine; D is dung. Waldrip et al. (2013). Vu et al. (2009a, b); Andersen et al. (2020); Haynes and Williams (1993); Marsden et al. (2020); Dong et al. (2014); Waldrip et al. (2013); Nahm (2003); Hoogendoorn et al. (2011); Choirunnisa et al. (2019); Zhao et al. (2016); Reed et al. (2015); Sommer and Hutchings (2001); Misselbrook et al. (2016); Selbie et al. (2015).
Table A2Divisions of manure management used in AMCLIM.
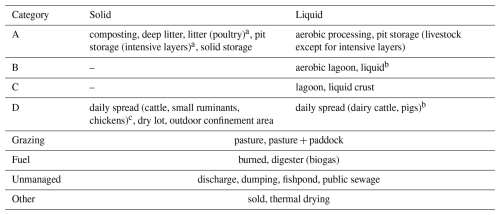
a Counted as housing emissions. b To differentiate from “liquid crust”, “liquid” is assumed to be an uncovered storage. c Counted as MMS emissions.
Table A3Information on US Environmental Protection Agency Animal Feeding Operations monitoring data.
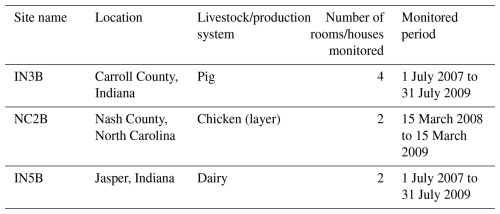
Table A4Model inputs for site simulations and global simulations. * The reference year of these data is 2010, and changes in livestock population and N excretion rates over time are based on the variations suggested by Lu and Tian (2017) to derive livestock data in the year 2018.
Figure A11Differences in meteorological variables, including annual mean temperature (2 m), precipitation, subsurface percolation flux and volumetric soil water content (0–14 cm), between 2010 and 2018.
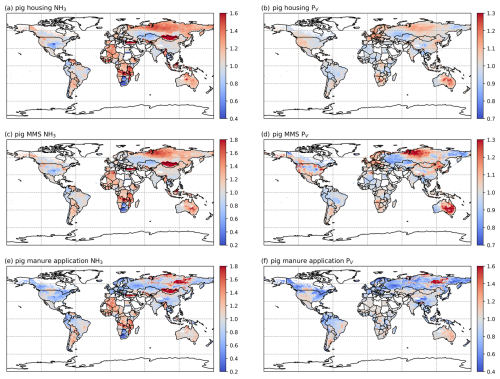
Figure A12Ratios of 2018 to 2010 NH3 emissions (a, c, e) and volatilization rates (PV) (b, d, f) from housing, manure management and manure application for pigs.
Model code can be obtained from GitHub (https://github.com/jjzwilliam/AMCLIM last access: 3 April 2024) and Zenodo (https://doi.org/10.5281/zenodo.10911886, Jiang, 2024). Model results presented in this study are in netCDF format and can be freely accessed from https://doi.org/10.7488/ds/7888 (Jiang et al., 2025).
The supplement related to this article is available online at https://doi.org/10.5194/gmd-18-5051-2025-supplement.
JJ, DSS and MAS designed the research. JJ developed the model, wrote the code, performed the simulations and wrote the paper. AU, GT, AF and FC provided model input data. All authors contributed to analysis and discussion of the model outputs, interpretation of results, writing, and critical revision.
The contact author has declared that none of the authors has any competing interests.
Publisher's note: Copernicus Publications remains neutral with regard to jurisdictional claims made in the text, published maps, institutional affiliations, or any other geographical representation in this paper. While Copernicus Publications makes every effort to include appropriate place names, the final responsibility lies with the authors.
Jize Jiang gratefully acknowledges support from the University of Edinburgh, UK Centre for Ecology and Hydrology (UKCEH); ETH Zurich; and the UK national supercomputing service ARCHER2.
This research has been supported by the UK Natural Environment Research Council (grant no. NE/S009019/2); by UKRI through the GCRF South Asian Nitrogen Hub, funded through the Global Challenges Research Fund; and by the ReCLEAN joint initiative, co-financed by the ETH Board. The authors are grateful for support from the Global Environment Facility (GEF) through the UN Environment Programme (UNEP) for the project “Towards the International Nitrogen Management System (INMS)”; from the UKRI under its Global Challenges Research Fund for support of the GCRF South Asian Nitrogen Hub (grant no. NE/S009019/2); from NERC for National Capability support, including through the CEH SUNRISE project; and from the ReCLEAN joint initiative at ETH Zurich under the ETH Board Joint Initiatives scheme.
This paper was edited by Bo Zheng and reviewed by two anonymous referees.
Aarnink, A. J. A., Swierstra, D., van den Berg, A. J., and Speelman, L.: Effect of Type of Slatted Floor and Degree of Fouling of Solid Floor on Ammonia Emission Rates from Fattening Piggeries, J. Agr. Eng. Res., 66, 93–102, https://doi.org/10.1006/jaer.1996.0121, 1997.
Andersen, H. M.-L., Kongsted, A. G., and Jakobsen, M.: Pig elimination behavior – A review, Appl. Anim. Behav. Sci., 222, 104888, https://doi.org/10.1016/j.applanim.2019.104888, 2020.
Aneja, V., Bunton, B., Walker, J. T., and Malik, B. P.: Measurement and analysis of atmospheric ammonia emissions from anaerobic lagoons, Atmos. Environ., 35, 1949–1958, https://doi.org/10.1016/S1352-2310(00)00547-1, 2001.
Ball, R., Keeney, D. R., Thoebald, P. W., and Nes, P.: Nitrogen Balance in Urine-affected Areas of a New Zealand Pasture 1, Agron. J., 71, 309–314, https://doi.org/10.2134/agronj1979.00021962007100020022x, 1979.
Beaudor, M., Vuichard, N., Lathière, J., Evangeliou, N., Van Damme, M., Clarisse, L., and Hauglustaine, D.: Global agricultural ammonia emissions simulated with the ORCHIDEE land surface model, Geosci. Model Dev., 16, 1053–1081, https://doi.org/10.5194/gmd-16-1053-2023, 2023.
Behera, S. N., Sharma, M., Aneja, V. P., and Balasubramanian, R.: Ammonia in the atmosphere: a review on emission sources, atmospheric chemistry and deposition on terrestrial bodies, Environ. Sci. Pollut. R., 20, 8092–8131, https://doi.org/10.1007/s11356-013-2051-9, 2013.
Bell, M., Flechard, C., Fauvel, Y., Häni, C., Sintermann, J., Jocher, M., Menzi, H., Hensen, A., and Neftel, A.: Ammonia emissions from a grazed field estimated by miniDOAS measurements and inverse dispersion modelling, Atmos. Meas. Tech., 10, 1875–1892, https://doi.org/10.5194/amt-10-1875-2017, 2017.
Bittman, S., Dedina, M., Howard, C. M., Oenema, O., and Sutton, M. A.: Options for ammonia mitigation: guidance from the UNECE Task Force on Reactive Nitrogen, Edinburgh, NERC/Centre for Ecology & Hydrology, 83 pp., https://www.clrtap-tfrn.org/ (last access: 6 August 2025), 2014.
Bouwman, A. F., Lee, D. S., Asman, W. A. H., Dentener, F. J., Van Der Hoek, K. W., and Olivier, J. G. J.: A global high-resolution emission inventory for ammonia, Global Biogeochem. Cy., 11, 561–587, https://doi.org/10.1029/97GB02266, 1997.
Bussink, D. W.: Ammonia volatilization from grassland receiving nitrogen fertilizer and rotationally grazed by dairy cattle, Fert. Res., 33, 257–265, https://doi.org/10.1007/BF01050881, 1992.
Bussink, D. and Oenema, O.: Ammonia volatilization from dairy farming systems in temperate areas: a review, Nutr. Cycl. Agroecosys., 51, 19–33, https://doi.org/10.1023/A:1009747109538, 1998.
Carran, R. A., Ball, P. R., Theobald, P. W., and Collins, M. E. G.: Soil nitrogen balances in urine-affected areas under two moisture regimes in Southland, New Zeal. J. Exp. Agr., 10, 377–381, https://doi.org/10.1080/03015521.1982.10427902, 1982.
Chantigny, M. H., Rochette, P., Angers, D. A., Massé, D., and Côté, D.: Ammonia volatilization and selected soil characteristics following application of anaerobically digested pig slurry, Soil Sci. Soc. Am. J., 68, 306–312, https://doi.org/10.2136/sssaj2004.3060, 2004.
Choirunnisa, R., Luthfi, N., Prima, A., Restitrisnani, V., Subagyo, W., Arifin, M., Rianto, E., and Purnomoadi, A.: Comparison of N excretion between Goat and Sheep, IOP Conf. Ser.-Earth Environ. Sci., 247, 012018, https://doi.org/10.1088/1755-1315/247/1/012018, 2019.
Dentener, F. J. and Crutzen, P. J.: A three-dimensional model of the global ammonia cycle, J. Atmos. Chem., 19, 331–369, https://doi.org/10.1007/BF00694492, 1994.
Dong, R. L., Zhao, G. Y., Chai, L. L., and Beauchemin, K. A.: Prediction of urinary and fecal nitrogen excretion by beef cattle, J. Anim. Sci., 92, 4669–4681, https://doi.org/10.2527/jas.2014-8000, 2014.
Elliott, H. A. and Collins, N. E.: Factors Affecting Ammonia Release in Broiler Houses, Trans. ASAE, 25, 0413–0418, https://doi.org/10.13031/2013.33545, 1982.
EMEP/EEA: EMEP/EEA Air Pollutant Emission Inventory Guidebook 2019 – Technical Guidance to Prepare National Emission Inventories, EEA Report No. 13/2019, https://www.eea.europa.eu/publications/ (last access: 26 March 2025), 2019.
FAO: Global Livestock Environmental Assessment Model, http://www.fao.org/gleam/en/, last access: 7 February 2018.
FAO: Nitrogren inputs to agricultural soils from livestock manure: new statistics, Food and Agriculture Organization of the United Nations, Rome, https://openknowledge.fao.org/items/0e1e7de9-aee1-4f29-a0a4-a59c8a78f82b (last access: 15 November 2024), 2022.
Fischer, K., Burchill, W., Lanigan, G. J., Kaupenjohann, M., Chambers, B. J., Richards, K. G., and Forrestal, P. J.: Ammonia emissions from cattle dung, urine and urine with dicyandiamide in a temperate grassland, Soil Use Manage., 32, 83–91, https://doi.org/10.1111/sum.12203, 2016.
Flechard, C. R., Massad, R.-S., Loubet, B., Personne, E., Simpson, D., Bash, J. O., Cooter, E. J., Nemitz, E., and Sutton, M. A.: Advances in understanding, models and parameterizations of biosphere-atmosphere ammonia exchange, Biogeosciences, 10, 5183–5225, https://doi.org/10.5194/bg-10-5183-2013, 2013.
Frank, D. A. and Zhang, Y.: Ammonia volatilization from a seasonally and spatially variable grazed grassland: Yellowstone National Park, Biogeochemistry., 36, 189–203, https://doi.org/10.1023/A:1005705121160, 1997.
Gyldenkærne, S.: A dynamical ammonia emission parameterization for use in air pollution models, J. Geophys. Res., 110, D07108, https://doi.org/10.1029/2004JD005459, 2005.
Haynes, R. J. and Williams, P. H.: Nutrient Cycling and Soil Fertility in the Grazed Pasture Ecosystem, Adv. Agron., 49, 119–199, https://doi.org/10.1016/S0065-2113(08)60794-4, 1993.
Hersbach, H., Bell, B., Berrisford, P., Hirahara, S., Horányi, A., Muñoz-Sabater, J., Nicolas, J., Peubey, C., Radu, R., Schepers, D., Simmons, A., Soci, C., Abdalla, S., Abellan, X., Balsamo, G., Bechtold, P., Biavati, G., Bidlot, J., Bonavita, M., Chiara, G., Dahlgren, P., Dee, D., Diamantakis, M., Dragani, R., Flemming, J., Forbes, R., Fuentes, M., Geer, A., Haimberger, L., Healy, S., Hogan, R. J., Hólm, E., Janisková, M., Keeley, S., Laloyaux, P., Lopez, P., Lupu, C., Radnoti, G., Rosnay, P., Rozum, I., Vamborg, F., Villaume, S., and Thépaut, J.: The ERA5 global reanalysis, Q. J. Roy Meteor. Soc., 146, 1999–2049, https://doi.org/10.1002/qj.3803, 2020.
Hoogendoorn, C. J., Betteridge, K., Ledgard, S. F., Costall, D. A., Park, Z. A., and Theobald, P. W.: Nitrogen leaching from sheep-, cattle- and deer-grazed pastures in the Lake Taupo catchment in New Zealand, Anim. Prod. Sci., 51, 416, https://doi.org/10.1071/AN10179, 2011.
Hutchings, N. J., Sommer, S. G., and Jarvis, S. C.: A model of ammonia volatilization from a grazing livestock farm, Atmos. Environ., 30, 589–599, https://doi.org/10.1016/1352-2310(95)00315-0, 1996.
Jarvis, S. C., Hatch, D. J., and Lockyer, D. R.: Ammonia fluxes from grazed grassland: annual losses from cattle production systems and their relation to nitrogen inputs, J. Agr. Sci., 113, 99–108, https://doi.org/10.1017/S0021859600084677, 1989a.
Jarvis, S. C., Hatch, D. J., and Roberts, D. H.: The effects of grassland management on nitrogen losses from grazed swards through ammonia volatilization; the relationship to excretal N returns from cattle, J. Agr. Sci., 112, 205–216, https://doi.org/10.1017/S0021859600085117, 1989b.
Jarvis, S. C., Hatch, D. J., Orr, R. J., and Reynolds, S. E.: Micrometeorological studies of ammonia emission from sheep grazed swards, J. Agr. Sci., 117, 101–109, https://doi.org/10.1017/S0021859600079028, 1991.
Jiang, J.: AMmonia-CLIMate (AMCLIM) v1.0, Zenodo [code], https://doi.org/10.5281/zenodo.10911886, 2024.
Jiang, J., Stevenson, D. S., Uwizeye, A., Tempio, G., and Sutton, M. A.: A climate-dependent global model of ammonia emissions from chicken farming, Biogeosciences, 18, 135–158, https://doi.org/10.5194/bg-18-135-2021, 2021.
Jiang, J., Stevenson, D. S., and Sutton, M. A.: A dynamical process-based model for quantifying global agricultural ammonia emissions – AMmonia–CLIMate v1.0 (AMCLIM v1.0) – Part 1: Land module for simulating emissions from synthetic fertilizer use, Geosci. Model Dev., 17, 8181–8222, https://doi.org/10.5194/gmd-17-8181-2024, 2024.
Jiang, J., Stevenson, D., and Sutton, M.: Data supporting the manuscript “A dynamical process-based model for quantifying global agricultural ammonia emissions – AMmonia–CLIMate v1.0 (AMCLIM v1.0) – Part 2: livestock farming”, 2010, University of Edinburgh. School of Geosciences. Global Change Group [data set], https://doi.org/10.7488/ds/7888, 2025.
Jørgensen, H., Prapaspongsa, T., Vu, V. T. K., and Poulsen, H. D.: Models to quantify excretion of dry matter, nitrogen, phosphorus and carbon in growing pigs fed regional diets, J. Anim. Sci. Biotechno., 4, 42, https://doi.org/10.1186/2049-1891-4-42, 2013.
Laubach, J., Taghizadeh-Toosi, A., Sherlock, R. R., and Kelliher, F. M.: Measuring and modelling ammonia emissions from a regular pattern of cattle urine patches, Agr. Forest Meteorol., 156, 1–17, https://doi.org/10.1016/j.agrformet.2011.12.007, 2012.
Laubach, J., Taghizadeh-Toosi, A., Gibbs, S. J., Sherlock, R. R., Kelliher, F. M., and Grover, S. P. P.: Ammonia emissions from cattle urine and dung excreted on pasture, Biogeosciences, 10, 327–338, https://doi.org/10.5194/bg-10-327-2013, 2013.
Lim, T. T., Chen, L., Jin, Y., Ha, C., Ni, J.-Q., Bogan, B. W., Ramirez, J. C., Diehl, C., Xiao, C., and Heber, A. J.: National Air Emissions Monitoring Study: Emissions Data from Four Swine Finishing Rooms – Site IN3B. Final Report, Purdue University, https://archive.epa.gov/airquality/afo2012/web/html/index.html (last access: 11 March 2020), 2010a.
Lim, T. T., Jin, Y., Ha, J. H., and Heber, A. J.: National Air Emissions Monitoring Study: Emissions Data from Two Freestall Barns and a Milking Center at a Dairy Farm in Indiana – Site IN5B, Purdue University, https://archive.epa.gov/airquality/afo2012/web/html/index.html (last access: 11 March 2020), 2010b.
Liss, P. S.: Processes of gas exchange across an air-water interface, Deep-Sea Res., 20, 221–238, 1973.
Liss, P. S. and Slater, P. G.: Flux of Gases across the Air-Sea Interface, Nature, 247, 181–184, https://doi.org/10.1038/247181a0, 1974.
Lockyer, D. R. and Whitehead, D. C.: Volatilization of ammonia from cattle urine applied to grassland, Soil Biol. Biochem., 22, 1137–1142, https://doi.org/10.1016/0038-0717(90)90040-7, 1990.
Lu, C. and Tian, H.: Global nitrogen and phosphorus fertilizer use for agriculture production in the past half century: shifted hot spots and nutrient imbalance, Earth Syst. Sci. Data, 9, 181–192, https://doi.org/10.5194/essd-9-181-2017, 2017.
Marsden, K. A., Lush, L., Holmberg, Jon. A., Whelan, M. J., King, A. J., Wilson, R. P., Charteris, A. F., Cardenas, L. M., Jones, D. L., and Chadwick, D. R.: Sheep urination frequency, volume, N excretion and chemical composition: Implications for subsequent agricultural N losses, Agr. Ecosyst. Environ., 302, 107073, https://doi.org/10.1016/j.agee.2020.107073, 2020.
Massad, R.-S., Nemitz, E., and Sutton, M. A.: Review and parameterisation of bi-directional ammonia exchange between vegetation and the atmosphere, Atmos. Chem. Phys., 10, 10359–10386, https://doi.org/10.5194/acp-10-10359-2010, 2010.
Misselbrook, T., Fleming, H., Camp, V., Umstatter, C., Duthie, C.-A., Nicoll, L., and Waterhouse, T.: Automated monitoring of urination events from grazing cattle, Agr. Ecosyst. Environ., 230, 191–198, https://doi.org/10.1016/j.agee.2016.06.006, 2016.
Misselbrook, T. H., Scholefield, D., and Parkinson, R.: Using time domain reflectometry to characterize cattle and pig slurry infiltration into soil, Soil Use Manage., 21, 167–172, https://doi.org/10.1111/j.1475-2743.2005.tb00121.x, 2006.
Móring, A., Vieno, M., Doherty, R. M., Laubach, J., Taghizadeh-Toosi, A., and Sutton, M. A.: A process-based model for ammonia emission from urine patches, GAG (Generation of Ammonia from Grazing): description and sensitivity analysis, Biogeosciences, 13, 1837–1861, https://doi.org/10.5194/bg-13-1837-2016, 2016.
Nahm, K. H.: Evaluation of the nitrogen content in poultry manure, World Poultry Sci. J., 59, 77–88, https://doi.org/10.1079/WPS20030004, 2003.
Nemitz, E., Milford, C., and Sutton, M. A.: A two-layer canopy compensation point model for describing bi-directional biosphere-atmosphere exchange of ammonia, Q. J. Roy. Meteor. Soc., 127, 815–833, https://doi.org/10.1002/qj.49712757306, 2001.
Pinder, R. W., Pekney, N. J., Davidson, C. I., and Adams, P. J.: A process-based model of ammonia emissions from dairy cows: improved temporal and spatial resolution, Atmos. Environ., 38, 1357–1365, https://doi.org/10.1016/j.atmosenv.2003.11.024, 2004.
Reed, K. F., Moraes, L. E., Casper, D. P., and Kebreab, E.: Predicting nitrogen excretion from cattle, J. Dairy Sci., 98, 3025–3035, https://doi.org/10.3168/jds.2014-8397, 2015.
Riddick, S. N., Blackall, T. D., Dragosits, U., Tang, Y. S., Moring, A., Daunt, F., Wanless, S., Hamer, K. C., and Sutton, M. A.: High temporal resolution modelling of environmentally-dependent seabird ammonia emissions: Description and testing of the GUANO model, Atmos. Environ., 161, 48–60, https://doi.org/10.1016/j.atmosenv.2017.04.020, 2017.
Robinson, T. P., Wint, G. R. W., Conchedda, G., Van Boeckel, T. P., Ercoli, V., Palamara, E., Cinardi, G., D'Aietti, L., Hay, S. I., and Gilbert, M.: Mapping the Global Distribution of Livestock, Plos One, 9, e96084, https://doi.org/10.1371/journal.pone.0096084, 2014.
Ryden, J. C., Whitehead, D. C., Lockyer, D. R., Thompson, R. B., Skinner, J. H., and Garwood, E. A.: Ammonia emission from grassland and livestock production systems in the UK, Environ. Pollut., 48, 173–184, https://doi.org/10.1016/0269-7491(87)90032-7, 1987.
Saarijärvi, K., Mattila, P. K., and Virkajärvi, P.: Ammonia volatilization from artificial dung and urine patches measured by the equilibrium concentration technique (JTI method), Atmos. Environ., 40, 5137–5145, https://doi.org/10.1016/j.atmosenv.2006.03.052, 2006.
Seedorf, J., Hartung, J., Schröder, M., Linkert, K. H., Pedersen, S., Takai, H., Johnsen, J. O., Metz, J. H. M., Groot Koerkamp, P. W. G., Uenk, G. H., Phillips, V. R., Holden, M. R., Sneath, R. W., Short, J. L. L., White, R. P., and Wathes, C. M.: A Survey of Ventilation Rates in Livestock Buildings in Northern Europe, J. Agr. Eng. Res., 70, 39–47, https://doi.org/10.1006/jaer.1997.0274, 1998.
Selbie, D. R., Buckthought, L. E., and Shepherd, M. A.: The Challenge of the Urine Patch for Managing Nitrogen in Grazed Pasture Systems, Adv. Agron., 129, 229–292, https://doi.org/10.1016/bs.agron.2014.09.004, 2015.
Seré, C. and Steinfeld, H.: World livestock production systems. FAO Animal Production and Health Paper 127, Rome, Italy: FAO, https://openknowledge.fao.org/server/api/ (last access: 15 November 2024), 1996.
Sherlock, R. and Goh, K.: Dynamics of ammonia volatilization from simulated urine patches and aqueous urea applied to pasture I. Field experiments, Fert. Res., 5, 181–195, https://doi.org/10.1007/BF01052715, 1984.
Sommer, S. G. and Hutchings, N. J.: Ammonia emission from field applied manure and its reduction – invited paper, Eur. J. Agron., 15, 1–15, https://doi.org/10.1016/S1161-0301(01)00112-5, 2001.
Sommer, S. G., Zhang, G. Q., Bannink, A., Chadwick, D., Misselbrook, T., Harrison, R., Hutchings, N. J., Menzi, H., Monteny, G. J., Ni, J. Q., Oenema, O., and Webb, J.: Algorithms Determining Ammonia Emission from Buildings Housing Cattle and Pigs and from Manure Stores, Adv. Agron., 89, 261–335, https://doi.org/10.1016/S0065-2113(05)89006-6, 2006.
Sommer, S. G., Webb, J., and Hutchings, N. D.: New emission factors for calculation of ammonia volatilization from European livestock manure management systems, Frontiers in Sustainable Food Systems, 3, 101, https://doi.org/10.3389/fsufs.2019.00101, 2019.
Sutton, M. A., Schjørring, J. K., and Wyers, G. P.: Plant-atmosphere exchange of ammonia, Philos. T. R. Soc. Lond., 351, 261–278, https://doi.org/10.1098/rsta.1995.0033, 1995.
Sutton, M. A., Howard, C. M., Erisman, J. W., Billen, G., Bleeker, A., Grennfelt, P., Van Grinsven, H., and Grizzetti, B. (Eds.): The European Nitrogen Assessment: Sources, Effects and Policy Perspectives, 1st edn., Cambridge University Press, https://doi.org/10.1017/CBO9780511976988, 2011.
Sutton, M. A., Bleeker, A., Howard, C. M., Bekunda, M., Grizzetti, B., de Vries, W., van Grinsven, H. J. M., Abrol, Y. P., Adhya, T. K., Billen G., Davidson, E. A., Datta, A., Diaz, R., Erisman, J. W., Liu, X. J., Oenema, O., Palm, C., Raghuram, N., Reis, S., Scholz, R. W., Sims, T., Westhoek, H., and Zhang F. S., with contributions from Ayyappan, S., Bouwman, A. F., Bustamante, M., Fowler, D., Galloway, J. N., Gavito, M. E., Garnier, J., Greenwood, S., Hellums, D. T., Holland, M., Hoysall, C., Jaramillo, V. J., Klimont, Z., Ometto, J. P., Pathak, H., Plocq Fichelet, V., Powlson, D., Ramakrishna, K., Roy, A., Sanders, K., Sharma, C., Singh, B., Singh, U., Yan, X. Y., and Zhang, Y.: Our Nutrient World: The challenge to produce more food and energy with less pollution. Global Overview of Nutrient Management, Centre for Ecology and Hydrology, Edinburgh on behalf of the Global Partnership on Nutrient Management and the International Nitrogen Initiative, https://nora.nerc.ac.uk/id/eprint/500700/ (last access: 28 October 2024), 2013.
Sutton, M. A., Howard, C. M., Mason, K. E., Brownlie, W. J., Cordovil, C. M. d. S. (Eds.): Nitrogen opportunities for agriculture, food & environment, UNECE guidance document on integrated sustainable nitrogen management, Edinburgh, UK Centre for Ecology & Hydrology, 157 pp., INMS Report 2022/02, https://nora.nerc.ac.uk/id/eprint/534033/ (last access: 28 October 2024), 2022.
Uwizeye, A., de Boer, I. J. M., Opio, C. I., Schulte, R. P. O., Falcucci, A., Tempio, G., Teillard, F., Casu, F., Rulli, M., Galloway, J. N., Leip, A., Erisman, J. W., Robinson, T. P., Steinfeld, H., and Gerber, P. J.: Nitrogen emissions along global livestock supply chains, Nat. Food, 1, 437–446, https://doi.org/10.1038/s43016-020-0113-y, 2020.
Vallis, I., Harper, L., Catchpoole, V., and Weier, K.: Volatilization of ammonia from urine patches in a subtropical pasture, Aust. J. Agr. Res., 33, 97, https://doi.org/10.1071/AR9820097, 1982.
Vallis, I., Peake, D., Jones, R., and McCown, R.: Fate of urea-nitrogen from cattle urine in a pasture-crop sequence in a seasonally dry tropical environment, Aust. J. Agr. Res., 36, 809, https://doi.org/10.1071/AR9850809, 1985.
Vira, J., Hess, P., Melkonian, J., and Wieder, W. R.: An improved mechanistic model for ammonia volatilization in Earth system models: Flow of Agricultural Nitrogen version 2 (FANv2), Geosci. Model Dev., 13, 4459–4490, https://doi.org/10.5194/gmd-13-4459-2020, 2020.
Voglmeier, K., Jocher, M., Häni, C., and Ammann, C.: Ammonia emission measurements of an intensively grazed pasture, Biogeosciences, 15, 4593–4608, https://doi.org/10.5194/bg-15-4593-2018, 2018.
Vu, T. K. V., Sommer, G. S., Vu, C. C., and Jørgensen, H.: Assessing Nitrogen and Phosphorus in Excreta from Grower-finisher Pigs Fed Prevalent Rations in Vietnam, Asian Australas, J. Anim. Sci, 23, 279–286, https://doi.org/10.5713/ajas.2010.90340, 2009a.
Vu, V. T. K., Prapaspongsa, T., Poulsen, H. D., and Jørgensen, H.: Prediction of manure nitrogen and carbon output from grower-finisher pigs, Anim. Feed Sci. Tech., 151, 97–110, https://doi.org/10.1016/j.anifeedsci.2008.10.008, 2009b.
Waldrip, H. M., Todd, R. W., and Cole, N. A.: Prediction of nitrogen excretion by beef cattle: A meta-analysis1,2,3, J. Anim. Sci., 91, 4290–4302, https://doi.org/10.2527/jas.2012-5818, 2013.
Wang, K., Kilic, K. I., Li, Q., Wang, L., Bogan, W. L., Ni, J.-Q., Chai, L., and Heber, A. J.: National Air Emissions Monitoring Study: Emissions Data from Two Tunnel-Ventilated Layer Houses in North Carolina – Site NC2B, Final Report, Purdue University, https://archive.epa.gov/airquality/afo2012/web/html/index.html (last access: 11 March 2020), 2010.
Whitehead, D. C.: Atmospheric ammonia in relation to grassland agriculture and livestock production, Soil Use Manage., 6, 63–65, https://doi.org/10.1111/j.1475-2743.1990.tb00802.x, 1990.
Yang, Y., Liu, L., Liu, P., Ding, J., Xu, H., and Liu, S.: Improved global agricultural crop- and animal-specific ammonia emissions during 1961–2018, Agriculture, Ecosyst. Environ., 344, 108289, https://doi.org/10.1016/j.agee.2022.108289, 2023.
Zhao, Y. G., Gordon, A. W., O'Connell, N. E., and Yan, T.: Nitrogen utilization efficiency and prediction of nitrogen excretion in sheep offered fresh perennial ryegrass (Lolium perenne), J. Anim. Sci., 94, 5321–5331, https://doi.org/10.2527/jas.2016-0541, 2016.